Introduction
Chemical products are used worldwide in a variety of industrial processes, both as intermediaries and as finished products. The evolution of the chemical industry toward the pharmaceutical sector has enabled an improvement in the quality of life of human beings thanks to the development of new drugs capable to treat or cure various conditions, but these compounds have also damaged the aquatic systems in the world.
At present, an alarming use of hormones and other products from various medical treatments or natural biological processes have been detected in aquifers, related with effects like the decrease in fertility, feminization, hermafrodismo, among others, in the aquatic fauna (Arlos et al., 2016; Cóppola, Nader & Aguirre, 2005; Han et al., 2012; Hansen et al., 1998; Lodhi & Killingbeck, 1982; Pelissero et al., 1993).
Estrone (E1) is a natural estrogen-type hormone which is, produced mainly by the ovaries, adipose tissue, fibroblasts skin, placenta, and brain (Cóppola et al., 2005). The E1 is present after menopause, however, its presence before menopause includes health problems, such as polycystic ovarian syndrome or cancer.
Estrone (E1) is part of the so-called endocrine disrupting compounds (EDC), a type of organic contaminants present at trace levels in the water that can interfere with the normal function of the endocrine systems in humans and wildlife (Alvarez-Corena, Bergendahl & Hart, 2016; Han et al., 2012; Sornalingam et al., 2018).
The presence of estrogens in drinking water has awakened the attention in the area of water treatment, as well as in the field of health due to its extremely high estrogenic power and its widespread presence in effluents from the wastewater treatment plant (WWTP) and in the receiving waters (Han et al., 2012; Zhang et al., 2012).
A conventional wastewater treatment plant (WWTP) usually does not completely eliminate the E1, mainly due to its resistance to biodegradation. In fact, an increase has been reported in the concentration of E1 after treatment of a wastewater effluent due to the formation of E1 as a by-product of degradation of the more powerful 17-P-estradiol (E2) (Sornalingam et al., 2018).
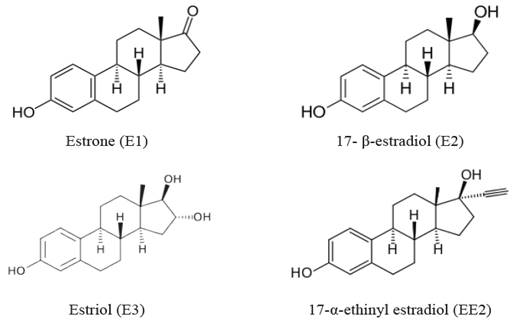
Figure 1 shows the molecular structures of the estrone (E1) and the rest of the natural female hormones; the 17-P-estradiol (E2) and unconjugated estriol (E3), as well as the synthetic hormone, the 17-α-ethinyl estradiol (EE2), while Table 1 shows the physical and chemical properties of these hormones, particularly the acid dissociation constant (pKa) and the octanol-water partition coefficient (log KOW). The E1 and the rest of the hormones are weak acids and moderately soluble, which is reflected by their high pKa value (Arlos et al., 2016) Solubility can be analyzed through the values of the octanol-water partition coefficient, which reflects the preference to solubilize in water or hydrophobic environments: The higher the partition coefficient, the more soluble in water the hormone will be. Another characteristic of these compounds is that they have a maximum absorption of photons at wavelengths in the range of279 and 293 nm (Arlos et al., 2016).
Table 1 Physical and chemical properties of the E1, E2, E3 and EE2
| Hormone | Type | Molecular mass (g/mol) | Log K to pH 5.0 | Solubility at 25°C in pure water (mg/L) | |
|---|---|---|---|---|---|
| E1 | Least abundant hormone | 270,37 | 3,30 | 10,33 | 1,30 |
| E2 | Primary hormone | 272,38 | 3,75 | 10,33 | 1,51 |
| E3 | Abundant during pregnancy | 288,38 | 2,54 | 10,33 | 0,12 |
| EE2 | Birth Control Pill | 296,40 | 3,63 | 10,33 | 9,20 |
Note: pKa: Acid dissociation constant, log Kow: octanol-water partition coefficient
Source: Reproduced from Arlos et al. (2016)
On the other hand, advanced oxidation processes (AOP) may be effective in removing EDC from water (de Liz et al., 2018). The photodegradation by photolysis and photocatalysis is considered to be an efficient process for the elimination of EDC (de Liz et al., 2018) to trace level, as E1 (Sornalingam et al., 2018).
The photocatalytic oxidation photocataly-sis is used as the only source of energy to ultraviolet radiation. Additionally, there is the presence of a catalyst or semiconductor, such as titanium dioxide (TiO2). The TiO2 is an attractive photocatalyst due to its high chemical stability, commercial availability and it is non-toxic characteristics (Sornalingam et al., 2018). The heterogeneous photocatalysis has gained attention as a promising technique that can be used as an alternative or as a complement to conventional treatment of wastewater, since it allows the mineralization of recalcitrant pollutants (including organic and inorganic compounds, metals and pathogens) into CO2, H2O, water and a small amount of simple inorganic compounds (Czech & Rubinowska, 2013). Photocatalysis starts with the formation of hydroxyl radicals, which have an oxidizing effect on chemical pollutants, and the oxidation process takes place on the surface of the catalyst (Garcés, Mejía & Santamaría, 2004). The band gap energy for TiO2 is approximately 3.2 eV, which suggests that UV light with a wavelength less than or equal to 387 nm can be used for excitation. However, the E1, E2, E3, and EE2 have a maximum absorption of photons at wavelengths that occur between 279 and 293 nm (Arlos et al., 2016).
Photocatalysis has been used in different studies to degrade EDC, such as E1, in both synthetic and natural waters under different experimental conditions, such as: the shape of the catalyst (free or modified) the wavelength of the radiation and the pH (Arlos et al., 2016; Czech & Rubinowska, 2013; Frontistis et al., 2012; Li et al., 2010; Mizuguchi et al., 2005; Nakashima et al., 2003; Sornalingam et al., 2018; Zhang et al., 2012).
The main contribution of the research is to show the effect of: (a) the concentration of the catalyst, (b) the wavelength of the radiation, (c) the pH, and d) the matrix of water that have been used in the removal of the E1, and in this way, relating these experiments conditions to the efficiency to remove the hormone. This information is useful in terms of optimizing time and resources for the EDC degradation, which is the basis for the development of future research studies. Therefore, the objective of this systematic review is to describe the experimental conditions that have given the best results in the removal of the estrone by photocatalysis in synthetic water and wastewater.
Materials and Methods
The search procedure of the research articles
For the development of this research, a search was conducted on relevant scientific literature, using Scopus and ScienceDirect databases based on their great impact at international level.
The research is based on the following question: What are the experimental conditions that have given the best results in the degradation or removal of estrone using photocatalysis in water samples in accordance with the systematic review of the literature? This research question settled the terms that served as descriptors in the databases mentioned above, these being the following: Degradation, removal, photodegradation, estrone, titanium dioxide, TiO 2 andphotocatalytic. After selecting the descriptors listed above, the Boolean operators were used to ensure the specificity of the research: [(Degradation OR removal OR photodegradation) AND estrone and (TiO2 OR titanium dioxide) AND photocatalytic].
The search was performed in October 14, 2018, and consisted of two steps: first, it was wide in both bases, the search could appear in all fields, considered all the years included all types of documents (original articles, reviews, books, book chapters, among others). Then, with the aim of conducting a more specific research, a second exploration was carried out, taking as an inclusion criterion that the search words should appear in the title, summary or key words and documents should be published only in "journal" and in the English language. Finally, the criterion for inclusion was applied meaning that the article should contain Introduction, Methodology, Results, Analysis, and Discussion of Results, being defined this as IMRAD format, in order to describe the experimental conditions of degradation and the description of the photocatalytic process of estrone. On the other hand, the exclusion criterion was the existence of another technique of removal coupled to the photocatalysis or a modification of the traditional oxidative technique. Table 2 shows the search criteria, as well as the inclusion and exclusion conditions.
Procedure for the analysis of research papers
The research papers were reviewed on an individual basis in order to remove and discriminate against the information needed to answer the research question. The extracted information was associated with the concentration of the catalytic converter, the wavelength of the radiation, pH, and the array of water, and in this way, relating these conditions to the percentages of removal of the hormone. Then, based on the experimental results presented in the articles, the best conditions to degrade or remove estrone were identified. Finally, the extracted information is shown in Table 3.
Results
The identification of the research papers was conducted in the databases as indicated the October 14, 2018. In a first search, 318 publications were obtained in Scopus and 79 in ScienceDirect. The initial exploration in both databases was wide; the search words could appear in all fields, considering all the years, and included all types of documents (original articles, reviews, books, book chapters, among others). Then, the second exploration was conducted by taking as an inclusion criteria that the search words should appear in the title, summary or key words and only articles in english were allowed. As a result, 14 documents were obtained in Scopus and 3 in ScienceDirect, for a total of 17 articles. From the above, a search for duplicates was carried out using the Manager of references Mendeley, resulting in 3 papers, for a total of 14 articles. Finally, we applied inclusion and exclusion criteria (Table 2) to remove articles that do not correspond with these results, and in this way, 08 original research papers were obtained and included in this systematic review (Figure 2).
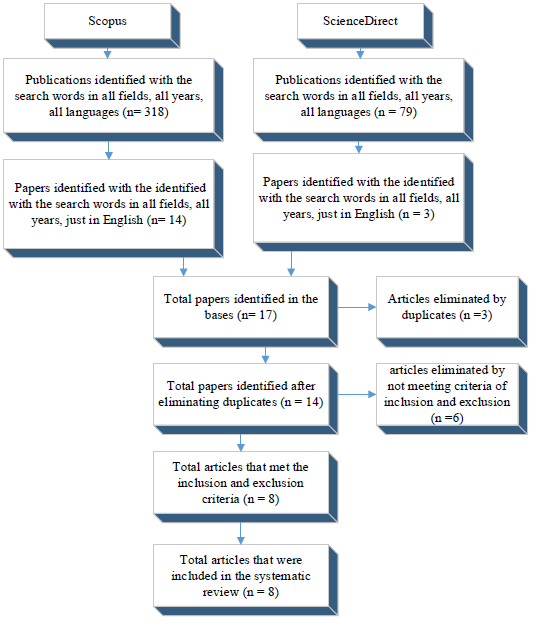
Source: elaborated by the authors
Figure 2 Search process of research papers on the efficiency of TiO2 photocatalysis to remove estrone from water.
On the other hand, out of the 08 publications used for a systematic review the results show that the country of origin of the articles are Australia (1), Canada (1), China (1), Greece (1), Japan (2), Poland (1), and United Kingdom (1), as shown in detail in Table 3.
Table 3 Articles by countries included in the systematic review
| Country | #Articles | Reference |
|---|---|---|
| Australia | 1 | (Sornalingam et al., 2018) |
| Canada | 1 | (Melles et al., 2016) |
| China | 1 | (Zhang et al., 2012) |
| Greece | 1 | (Frontistis et al., 2012) |
| Japan | 2 | (Mizuguchi et al., 2005; Nakashima et al., 2003) |
| Poland | 1 | (Czech & Rubinowska, 2013) |
| United Kingdom | 1 | (Li et al., 2010) |
Source: elaborated by the authors
Discussion
The analysis of the 08 research articles on the experimental conditions (concentration of the estrone, form the catalytic converter, the concentration of the catalyst, UV, pH conditions) that have been used in removal of estrone using photocatalysis, as well as the conversion of the hormone reached in the above-mentioned conditions, as shown in Table 4.
Table 4 Literature review on the experimental conditions used to remove estrone using photocatalysis
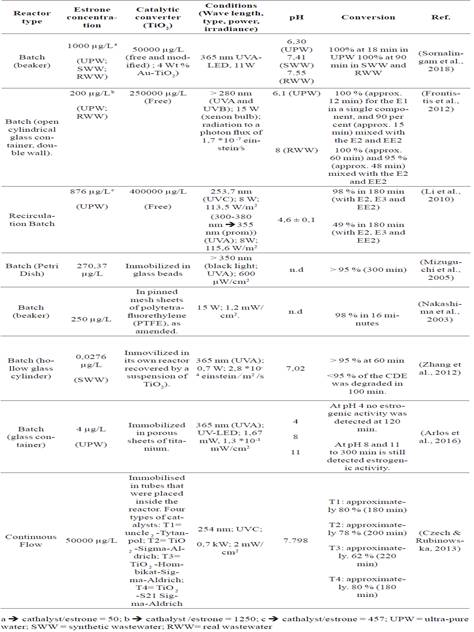
Source: elaborated by the authors
Effect of the load of the catalyst (TiO 2 )
In the research articles employed for the review notes, TiO2 was used preferably in immobilized form instead of its modified or free state. Thus, TiO2 was presented in porous titanium sheets (Arlos et al., 2016) in sheets of mesh of modified polytetrafluoroethylene (PTFE) (Nakashima et al., 2003), in glass beads (Mizuguchi et al., 2005), in the glass reactor itself with double coating (del TiO2) (Zhang et al., 2012) or immobilized in aluminum tubes through which the polluting solution passed (Czech & Rubinowska, 2013). The free TiO2 and modified with gold (Au) form were employed by (Sornalingam et al., 2018), where it was noted that, in general, the nanoparticles in 4% by weight ofAu-TiO2 presented a superior performance compared with TiO2 P25 in water and wastewater. The catalytic converter in its free form was only used by (Li et al., 2010) and (Frontistis et al., 2012).
The rate of photocatalytic degradation of E1 linearly increases as the concentration of the catalytic converter increases, which is consistent with the results of (Frontistis et al., 2012; Li et al., 2010 and Sornalingam et al., 2018). Table 4 shows the ratio catalyst/ estrone used by these researchers. It can be seen that the concentration of the catalyst was 1250, 457 and 50 times higher than the concentration of estrone as used by (Frontistis et al., 2012), (Li et al., 2010) and (Sornalingam et al., 2018), respectively. Estrone was completely removed in just 12 minutes when present in a simple matrix formed by the hormone and ultra-pure water, while a removal of 90 %> was achieved when a mixture of E1, E2 and the EE2 was used instead (Frontistis et al., 2012). According to Li et al. (2010) who used a ratio of catalyst/estrone of 457, a removal of 49 % of estrone was achieved after 180 minutes of treatment in ultra-pure water.
It is interesting to the analysis of the workload of the catalyst in (Sornalingam et al., 2018) research, where the catalyst/estrone reason is 50, but the total degradation of the present ultra-pure water E1 is reached in just 18 minutes. This result is linked to the modification of the catalyst, the aggregation of 4 % by weight of gold nanoparticles. These studies (Frontistis et al., 2012; Li et al., 2010; Sornalingam et al., 2018) evidence two things: firstly, the higher the concentration of the catalyst, the higher the degradation of the E1 will be, and secondly, a modification (dopping) of the catalyst may lead to improve its catalytic properties and overall effectiveness.
On the other hand, in the studies by Arlos et al. (2016) ; Czech & Rubinowska (2013) ; Mizuguchi et al. (2005) ; Nakashima et al. (2003) ; Zhang et al. (2012) the catalyst was used in an immobilized way, although the concentration of the catalyst is not clearly stated in the reports, it is known that when a catalyst is immobilized it increases its concentration and catalyst losses are reduced (Frontistis et al., 2012; Li et al., 2010; Sornalingam et al., 2018).
Effect of the wavelength of the radiation
The UV radiation is divided into UVC light (200-290 nm), UVB (290-320 nm) and UVA (320-400 nm). The preferred wavelength by research studies is 350-365 nm (UVA), and 254 nm (UVC), this under the premise that since the band gap energy for TiO2 is approximately 3,2 eV, suggesting that UV radiation with a wavelength less than or equal to 387 nm can be used for excitation, however, E1, E2, E3 and EE2 have a maximum absorption of photons in wavelengths that occur between 279 and 293 nm (Arlos et al., 2016).
The work presented by Li et al. (2010) degrades the E1 (in mixture with E2, E3 and EE3) under the same conditions of concentration of E1, catalyst concentration and pH, but the wavelength of the radiation is varied (the power of the lamp is the same and the flow of photons remains practically constant). In this way, a 98 % removal of E1 was achieved in 180 minutes using radiation at 253,7 nm (UVC), while under a radiation whose average was 355 nm, the removal in the same time was 49 %. This indicates that UVC radiation significantly increases the rate of degradation of a mixture of female hormones; (E1, E2, E3 and EE2) especially E1, given that the conversion of the rest of the hormones at the afore mentioned time was close to 60 %.
In the research studies conducted by Czech & Rubinowska (2013) UVC radiation was also used with different catalysts surface areas: (T1, T2, T3, and T4), achieving conversion percentages of 80 %, 78 %, 62 %, and 80 % in 180, 200, 220, and 180 minutes, respectively. However, estrone was the only hormone present in the analysis system, unlike the work presented in (Li et al., 2010).
Effect of pH
Arlos et al. (2016) reported the effect of pH on the degradation of E1 and other EDC. In this investigation, the effect of pH (4.0, 8.0 and 11.0) on the elimination of several EDC (including E1) was verified using UV-LED (UVA, 365 nm) and TiO2 was immobilized in porous titania sheets. At pH 4.0, no estrogenic activity was detected after 120 minutes of photocatalysis, while at pH values of 8.0 and 11.0, estrogenic activity was still detected after 300 minutes of treatment. The authors of (Arlos et al., 2016) indicate that the effect of pH on degradation is largely attributed to the charge on the surface of TiO2 and the degree of ionization of EDC in acidic or basic media. The surface of TiO2 is amphoteric and, as a result, different charged species are produced under different pH conditions. It should be understood that purely electrostatic interactions occur between the surface of the catalyst and the EDC, that is, there will be a greater or lesser interaction between them as reflected in the conversion of the EDC (including the E1).
At a pH of 4.0, the catalyst surface is mostly positive, which is indicated by the isoelectric point (IEP) value of 6.0 obtained by the catalyst (Arlos et al., 2016). Having the following condition (pH <IEP), and on the other hand, the EDC are mostly neutral, which favored the conversion of the EDC compared to the degradation obtained with the other pH values (8.0 and 11.0), where the catalyst surface is negatively charged and the EDC are mostly neutral (pH: 8.0) or have been ionized forming the corresponding anions, causing repulsion between them and thus affecting the rate of degradation.
Czech & Rubinowska (2013) reported that the initial pH of the solution containing E1 was 7,80 and that it decreased to 7,0, and although they did not describe the characteristics of the catalysts studied, they indicated that the adsorption of the contaminant had electroneutral characteristics (Czech & Rubinowska, 2013), that is, the pH was equal to the value of the point of zero charge (pH= PZC).
In summary, it is understood that the pH of the solution affects the rate of removal of the E1, since the catalyst surface and target substances are sensitive to the pH adjustments (Arlos et al., 2016; Czech & Rubinowska, 2013).
Effect of the water matrix
Frontistis et al. (2012) and Sornalingam et al. (2018) used several water matrices and analyzed the effect of this condition in the removal of E1. Frontistis et al.(2012) analyzed the removal of the hormone in ultra-pure water (UPW) and real waste water (RWW) using E1 as a single component and in a mixture with E2 and EE2. The results showed that when E1 was used alone, a greater removal was achieved in shorter contact times for both the pure UPW and the RWW. In contrast, when E1 was in a mixture, the degradation was slower, indicating that the competition occurred among the hormones (as well as the other non-target substances that were present in waste water) for the species responsible for the oxidation.
Sornalingam et al. (2018) used three matrices, UPW, SWW, and RWW were used. According to this results, the removal rate of E1 had the following order UPW > SWW ~ RWW; reaching a complete elimination of the hormone in UPW after 18 minutes and in SWW and RWW after 90 minutes of treatment. The above shows that the rate of removal of this hormone is influenced by the physical-chemical characteristics of the water in which estrone is found. For example, UPW is a simple matrix characterized by low conductivity, low turbidity, low chemical demand for oxygen, no ions or metals or alkalinity and no organic or inorganic carbon, among others. This means that there is no competition between the degradation target compound, E1 and the rest of the present compounds (organic or inorganic).
Conclusions
The rate of removal of E1 is strongly influenced by the experimental conditions at which photocatalysis is conducted. From the analysis of the scientific literature, it is observed that the removal of the E1 depends on the catalyst concentration and a high catalyst concentration means a greater removal. Also, a modification (dopping) of the catalyst may lead to improve its catalytic properties and overall effectiveness, for example; with the aggregation of 4 %> by weight of gold nanoparticles a total degradation of E1 was obtained in just 18 minutes. For its part, the use of radiation at 254 nm is convenient in matrices that contain mixtures of compounds (i.e. E1, E2, E3 and EE2), while the use of radiation at 365 nm gives good results provided that the pH of the solution is adjusted to a value lower than the isoelectric point (pH <IEP) or to the point of charge zero of the catalyst (pH = PZC). Finally, the removal rate is faster in UPW than in SWW and RWW and is influenced by the physicochemical characteristics of the water in which the E1 is located. In UPW, there is no presence of other compounds, therefore, there is no competition between the target compound of degradation -the E1- and the rest of the present compounds (organic or inorganic), leading to a higher removal rate in UPW than in SWW or RWW.