Introduction
Welding is the process of permanent bonding of two or more materials together, usually metals, by heat or pressure or both. When heated, the material reaches the molten state and can be joined together with or without adding additional fillers.
Many different energy sources can be used for welding including gas flames, electric arcs, electrical resistance, lasers, electron beams, friction, molten metal baths and ultrasounds. Welding includes joining methods as diverse as fusion, forging, friction, brazing, and blast. Welding is a potentially dangerous activity, so precautions are required to prevent electrocution, fire and explosion, burns, electric shock, visual damage, inhalation of poisonous gases and vapors and exposure to intense ultraviolet radiation. (Safe Work Australia, 2016)
Establishing different risks associated with welding is a very complex and difficult task, because currently there are no consolidated data from government agencies. However, its impact on productivity and the economy is such that according to the Swedish Welfare Commission estimates, in Europe there are 750,000 full-time workers in this activity, while in the US there are around 380,000 workers (Bureau of Labor Statistics, 2016), excluding those economies that have an undetermined number of workers in conditions of uncertain safety and hygiene such as China, India and many emerging countries. According to the above, the probability of acquiring occupational diseases, especially of the respiratory type, increases. Although the risks of welding are well characterized, the chronic effects related to exposure to metal fumes are not very clear yet (Szram, 2012).
Some reports of associated diseases include fever of the welder (Ahsan, 2009), siderosis (Cosgrove, 2015), susceptibility to pneumonia (Suri, 2016) and lower proportions of asthma (Health and Safety Executive, 2015), pulmonary cancer (Amboise, 2006; Sorensen, 2007); and the development of Parkinson's disease has also been related (Mortimer, 2012); while other studies confirm the prevalence of chronic bronchitis in workers, although it is not a general condition.
For welding processes, a variety of technologies are used, which have in common the use of metals to join the pieces, such as Cd, Cr, Ni, Mn, Pb, among others. During this union, fumes are formed, which vary their chemical composition and therefore their physicochemical properties, according to the materials used. This emitted material can be found to very fine particles and nanoparticles (Blamey,2016), which can lead to serious health disorders, individually orjointly (IARC, 2017).
Metal fumes
Metal fumes are a complex mixture of metal oxides, silicates and fluorides (CCOHS, 2016). This aerosol is generated when the molten metal is transferred to the base metal part at a higher temperature than the boiling point of its components, forming the vapors, which are condensed into very fine particles (suspended solids). Particle composition generally contains electrode and the material that is melting (Taube, 2013).
According to Table 1, the most common industrial method is Protected Arc Welding (SMAW), followed by Arc and Gas Arc Welding (GMAW), Submerged Arc Welding (SAW/ FCAW) and finally, welding of Tungsten with Electric Arc and Gas (GTAW).
Table 1 Welding processes and applications
| Process | Common Names or Synonyms | Applications |
| SMAW | Protected Electric Arc Welding Bar Welding Metal Welding with Electric Arc | Casting of ferrous metals or derivatives |
| GMAW | Welding with Submerged Arc Metal welding with inert gas Active gas metal welding | High Quality Welding that melts all metals and alloys in the industry Does not produce slag when welding |
| SAW | Submerged Arc Welding | Deep penetration High metal deposition rates |
| GTAW | Tungsten welding with inert gas | High Quality Welding that melts all metals and alloys in the industry Does not produce slag |
Source: Adapted from Eriksson, 2011.
Spray composition
The chemical composition of the metallic smoke generated in the welding process is very varied, given the amount of materials used, the technology and the addition of extra materials for the needs of a given process. Even so, the groups of substances that are part of the aerosol formed can be identified, which are the Volatile Organic Compounds (VOCs) and metal oxides.
Volatile Organic Compounds (VOCs)
The term volatile organic compounds (VOCs) includes a group of hydrocarbons that, under normal circumstances, are in gaseous form at room temperature, or have a high volatility under these conditions. European regulations define them as organic compounds with a vapor pressure of less than 0.01 kPa to 293.15 K. (Council of the European Union, 1999).
They are light compounds, with less than 12 carbon atoms, and various functional groups. There are more than one thousand compounds that can be considered VOCs; some of the VOCs found are methyl methacrylate, Acetaldehyde, CO2, NOx, ethanol, benzene, xylene, and toluene (Antonini, 2017, Safe Work Australia, 2016). Volatile organic compounds have characteristic properties responsible for their effects on health and the environment. Some of those properties are liposoluble, toxic and flammable compounds (IARC, 2017).
Metal Oxides
The content of metals in the aerosol is related to the welding process, regardless of the technology used to join the pieces. All these processes need such a huge quantity of energy that its principal effect is a sudden and immediate oxidation of the metal pieces. So inhalation of these fumes could cause misbalance on metal homeostasis.
Biologically, metals play a very important role in a variety of processes. The balance of metal ions, conserved through mechanisms of capture, storage and secretion, is critical for the conservation of the life of any organism; for this reason, it is required to maintain their levels (Bertini & Carvallaro, 2008). Some metals participate naturally in the organism in oxidation-reduction reactions, where they transfer electrons between two chemical species. These reactions are fundamental for the defense of organisms before the reactive oxygen species (ROS), which are transformed to less dangerous species by enzymes such as Superoxide Dismutase (SOD) (Zhou, 2015), where iron and copper have a participation fundamental in the functioning of said metalloproteins.
Alteration of the balance of metal ions can have consequences for the functioning of some systems in the organism; for example, in the case of proteins (enzymes, coenzymes), which naturally have metals attached to active sites, their functionality can be altered if they are replaced on the other (Nelson, 1999). On the other hand, there is enough evidence to establish that heavy metals can cause damage to DNA and proteins, causing a series of diseases (Valko, 2011). For example, excess iron in the body can cause cell damage by the formation of free radicals (superoxide, peroxide, hydroxyl). In addition, the alteration of the balance of copper and iron is a key element in the etiology of neurological diseases such as Alzheimer's and Parkinson's (Bush, 2008).
On the other hand, some other metals present in metallic smoke, such as Cadmium (Cd), Lead (Pb) and Chromium (Cr), do not have any known function in the body and present an important toxicity. These metals are also known as Heavy Metals, which refers to specific properties that distinguish them from other metals; for example, having a density of more than 5 g/cm3, in addition to generating effects in the environment and in living organisms at very low concentrations (Jarup, 2003).
Oxidative stress
Reactive oxygen species (ROS) are produced by living organisms as a normal result of cellular metabolism, especially in cellular respiration processes. On the other hand, oxidative stress occurs when the formation of the species exceeds the ability of the cell to neutralize them. Among the most relevant reactive oxygen species, Hydroxyl radical (OH.), superoxide anion (O2 -) and hydrogen peroxide (H2O2) are found. At low and moderate concentrations, the cell can function normally, but at high concentrations this can produce adverse effects on cellular components such as lipids, proteins and DNA (Valko, 2011). Homeostasis provides defense mechanisms against the production of these species by antioxidants that have the capacity to transform ROS into other less harmful ones, such as the enzymes Superoxide Dismutase (SOD) (McCord and Fridovich, 1969), Catalases and Glutathione Peroxidase (GPx). The SODs have within their molecular structure metals such as Copper, Iron, Manganese and Zinc, which by their ability to particípate in redox reactions play an important structural role. The oxidizing chemical species present in the cell are described below.
Table 2 Chemical species present in oxidative stress
| ROS | Description |
|---|---|
| O2 - Superoxide Anion | State of reduction of an electron of O2, formed in many reactions of auto oxidation and by the electron transport chain. It is not very reactive, but it can release Fe2+ from ferrosulfurized proteins and ferritin. Suffers dismutation to form H2O2 spontaneously or by enzymatic catalysis and is a precursor for the formation of • OH catalyzed by metals. |
| H2O2 Hydrogen peroxide | State of reduction of two electrons, formed by the dismutation of • O2 - or by direct reduction of O2. Soluble in lipids and therefore capable of diffusing through membranes. |
| • OH, Hydroxyl radical | State of reduction of three electrons, formed by the Fenton reaction and the decomposition of peroxynitrite. Extremely reactive, it attacks most cellular components. |
| ROOH, Organic hydroperoxide | Formed by radical reactions with cellular components such as lipids Y nucleobases. |
| RO •, alkoxy- and ROO •, peroxy- | Organic radicals centered on oxygen. Lipid forms participate in reactions of lipid peroxidation. Produced in the presence of oxygen by addition of radicals to double bonds or elimination of hydrogen. |
| HOCl, Hypochlorous acid | Formed from H2O2 for myeloperoxidase. Soluble in lipids and highly reactive. Rapidly oxidizes protein constituents, including thiol groups, amino groups Y methionine . |
Source: Adapted from Sharma etal., 2014.
The dismutation of the ROS starts with the transformation of the anion Superoxide O2 -., which is formed in cellular respiration processes within the mitochondria, to hydrogen peroxide (H2O2) and oxygen O2(Flora, 2009). Equation No. 1 shows this reaction.
O2 + (SOD)---(H 2O2+O2
Subsequently, the H2O2 formed is reduced by other SODs and glutathione peroxidase (GPX) enzymes thus decreasing oxidative stress (Hlavaty, 2000). This process is carried out by means of 2 stages, one Via Fenton and the other Via Haber Weiss, which in both cases culminate with the formation of the hydroxyl radical, as shown in Figure 1.
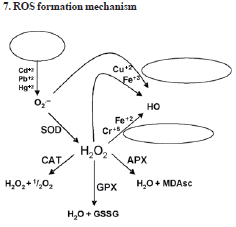
Source: Adapted from Pinto et al., 2003.
Figure 1 Generation of reactive oxygen species (ROS) by heavy metals.
Effects of ROS on the body
The effect and type of ROS that is formed are very varied according to the heavy metal to which body is exposed. Studies on the toxicity of heavy metals have been carried out investigating the effect of toxicity and carcinogenesis, since it is known that these alter biological systems inducing modifications in the nitrogenous bases of DNA (Flora, 2009), destruction of the lipid bilayer by peroxidation and protein damage (Valko, 2011).
With regard to carcinogenesis studies, it has been shown that ROS can cause damage to DNA and consequently to the cell, which begins to experience alterations in its life and death cycles. Cell death or Apoptosis is precisely a defense mechanism when the cell has lost all functionality for any reason, among them the excess of ROS. But when the concentration of these species is very high, they damage the DNA causing a disconnection of the cell at the central level and thus the cell only concentrates on duplicating itself uncontrollably. This process is known as neoplasia, and when the reproduction reaches a considerable size, cancerous tumors are formed (Flora, 2009).
Heavy metals and their relationship to the formation of ROS
Below we describe some of the most relevant chemical properties of metals such as Cr, Fe, Cd, Pb and Mn, their relationship with the formation of ROS and the possible effects on the health of exposed workers.
Cadmium (Cd)
Cadmium is a metal found in the earth's crust. According to the Agency for Toxic Substances and the Disease Registry (ASTDR, 2016), it is one of the most toxic heavy metals. This is obtained as a secondary product of mining processes associated with Zinc, Lead and Copper. For the metal-mechanic industry it is estimated that 7% of world production uses it, especially in electrolytic coatings (Mutlu, 2012).
Entrance of Cadmium to the organism occurs by inhalation of metallic smoke and this later passes to the blood, accumulating in the kidneys and liver. Its elimination is almost null causing chronic diseases due to its toxicity (ASTDR, 2016). It is important to note that the TLV for Cd is 0.01 mg / m3 per inhalation in fumes (OSHA, 2004). In addition, according to IARC, it belongs to group No. 1 as carcinogenic substance (IARC, 2017)
Among the most harmful effects of Cadmium is its ability to affect the enzymatic system of cells and generate oxidative stress. This occurs because Cd replaces Ca in the metalloproteins inhibiting their free radical protective activity, which causes damage due to the formation of ROS (Irfan, 2013). In addition, there is important evidence that Cadmium affects blood pressure, generating hypertension problems (Poreba, 2010).
Manganese (Mn)
Manganese is a grayish white transition metal very abundant in nature in different oxidation states ranging from +1 to +7. This variety of oxidation states allows Manganese to participate in oxidation-reduction reactions. On the other hand, its hardness and fragility stand out among its physical properties, meanwhile in chemical properties is when Mn forms compounds in their maximum state of oxidation (+7), since it is a very strong oxidizing agent employed in organic chemistry. In addition, Manganese is a trace element, and traces are essential for living organisms forming part of the enzyme Superoxide Dismutase (Mn-SOD) that catalyzes the reactive oxygen species (ROS) that form inside the cell (Miriyala, 2011).
Manganese is found in the metalworking industry in mild steels and steel alloys in order to improve their metallurgical properties by neutralizing the presence of sulfur and preventing the entry of oxygen into the molten metal (Barceloux, 1999). At the time of welding, approximately 15 % of the electrode's Mn is lost, becoming part of the metallic smoke, in which the presence of Mn compounds can vary from 0.2 % to 10 %, depending on the technology and electrode used (Taube, 2012)
Given its easy conversion to smoke, it represents a risk for exposed workers, because the inhalation of manganese dust is harmful to health and may be the cause of several clinical conditions. Since after its absorption in the body it crosses the pulmonary barrier, it reaches the blood and accumulates in the brain, causing adverse effects on the nervous system by blocking the neurotransmitters. The maximum amount allowed in the industry is 5 mg/m3 of air (NIOSH, 2008).
Manganese inhalation alters iron homeostasis causing the measurement of Mn in the blood of workers to be different form that in the target tissues. (Zheng, 2011). On the other hand, it has been related to different pathologies such as welder fever, Parkinson's disease, asthenia, chest pain, vomiting and kidney damage. (NIOSH, 2008). That is why future studies should carry out an adequate follow-up to confirm that manganese inhalation is directly related to all these mentioned diseases.
Chrome (Cr)
Chromium is the seventh most abundant element in nature (Mohanty and Kumar Patra, 2013). Chromium is a hard, brittle transition metal, gray and bright white, and it is very resistant against corrosion. Its highest oxidation state is +6, although these compounds are very oxidizing. Oxidation states +4 and +5 are rare, while the more stable states are +2 and +3. It is also possible to obtain compounds in which Chromium is present in lower oxidation states, but these are quite rare.
This element is widely used in metallurgy to improve resistance to corrosion and provide the characteristic bright color. This abundant use of the metal generates a significant increase in the pollution of Chromium affecting the environment and living species (Ghani, 2011). On the other hand, the most common Cr chemical species are Cr (III) and Cr (VI), the latter being toxic to humans (Gürkan, 2017). It has been established that the level of exposure of Cr (VI) by inhalation should not exceed 0.001 mg Cr (VI) / m3 (NIOSH, 2007) in a maximum of 10 hours.
Among the most relevant effects of inhalation is the reaction between Cr (VI) and thiols and ascorbates resulting in an increase in ROS. Cr (III) is not taken into account in the reactions as it is much less dangerous than Cr (VI) (Jaishankar, 2014). Finally, Cr (VI) is classified as a mutagenic and carcinogenic agent (IARC, 2011).
Lead (Pb)
Lead is a heavy metal with a relative density of 11.4 at 16 °C, it is silver-colored with bluish tint, which becomes cloudy to acquire a dull gray color. It is flexible, inelastic and melts easily. Its melting occurs at 327.4 °C and it boils at 1725 °C. The normal chemical valences are +2 and +4.
Lead is used in welding as a coating material due to its low melting point and malleability (American Federation of State, County & Municipal, 2011). It is an extremely toxic heavy metal, which in plants can cause damage to the chlorophyll and the photosynthetic process suppressing their growth (Najeeb, 2014; Mostafa, 2012). Furthermore, it has no known biological function whatsoever. Its exposure limit (TLV) to the inhalation of its vapors in the welding fumes is 0.05 mg/m3 (ACGIH, 2001).
There is a great variety of diseases associated with exposure to this heavy metal, whose main route is by inhalation. It is established that Lead can generate miscarriages in pregnant women (ATSDR, 2007), damage to the nervous system and kidneys, anemia, infertility in men, increase in blood pressure, brain cancer (Wu, 2012) and tremors (Isha, 2017 NTP, 2012). It has also been shown to have the ability to increase ROS formation causing increased oxidative stress causing lipid peroxidation (Wadhwa, 2012). In addition, poisoning with Pb decreases the activity of GPx at the brain level, since it reacts with the thiolic fragment of the enzyme. On the other hand, it can cause disruption of cell metabolism by having the ability to replace Ca+2, Mg+2, Fe+2 and Na+ metal cations in biological systems given the Pb ion mechanism (Flora, 2012).
Iron (Fe)
Iron is the fourth most abundant element in the earth's crust, representing 5% and, among metals, only aluminum is more abundant. It is the most abundant planetary mass, because the planet concentrates in its core the largest mass of native iron, equivalent to 70% (Garritz, 1998). Its most common valences are +2 and +3, allowing it to participate in reactions of type redox and managing to exchange their states (Phippen, 2008). It is an essential metal in the life of any organism and is part of many biological systems; its presence in proteins of the heme group is highlighted, such as hemoglobin, which is responsible for the transport of oxygen and carbon dioxide in the blood. It is also part of the catalases and peroxidases (Medline Plus, 2016). Despite the fact that there is an abundance of this metal in food, almost a third of the world population suffers from anemia (iron deficiency) (Medline Plus, 2016).
In welding processes, iron is a fundamental part in the composition of all steels (Fe / C), it is used as a metallic base in the design and construction of structures, and it is part of the electrodes used in the different processes (Sigma- Aldrich, 2017). Occupational exposure limit (TLV) for metal fumes is 10 mg / m3 (ACGIH, 2001).
In terms of occupational diseases associated with Iron, the most prevalent is siderosis (Billings and Howard., 1993). It also causes irritation in the nostrils, throat and lungs (Lay et al., 2001) and excess iron intracellularly can cause increased oxidative stress by increasing ROS, forming hydroxyl radicals (OH) and peroxyl (ROO.), that could lead to others ills (Prousek, 2007; Flora, 2009).
Conclusion
Exposure to heavy metals generated by metal fumes can generate a significant number of occupational diseases, which generate a large impact due to the number of workers engaged in welding, hygienic conditions and the volume of pollution generated.
Furthermore, the volume and chemical composition of metal fume is varied according to the techniques and materials used, but the danger of exposure continues to be high, since the slightest variation concentration of these metals in the body can trigger adverse effects related to oxidative stress and its subsequent reactions.
Finally, it is necessary to continue studying the size of the particle emitted, its composition and especially the effects of water solubility in order to be able to establish its effects in the organism














