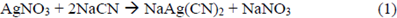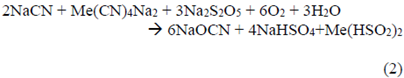1. Introduction
Since 1887, CN- is used in the hydrometallurgy industry, to extract gold and silver in alkaline conditions from the mineral ore containing them, as well as a reagent at low concentrations for the flotation of minerals such as lead, copper and zinc. The mining industry uses about 18% of the total CN- production [1] [2] [3] [4] [5] [6]. There are different processes to treat the effluents contaminated with CN-, such as biodegradation, alkaline chlorination, INCP process (SO2/air) (International Nickel Company), hydrogen peroxide, Caro's acid method (H2SO4/H2O2), ozonization, reverse osmosis, activated carbon, resins, among others [3] [6] [7] [8] [9] [10].
For the leaching of gold-containing ores using CN-, this may exist in three forms: total cyanide (CNT), weak acid dissociable cyanide or WAD and CNL [3-11]. The CNT includes the strong complexes such as iron cyanides ([Fe(CN)6]3 -, [Fe(CN)6]4 -), gold cyanides ([Au(CN)2]- and cobalt cyanides ([Co(CN)6]4 -) and WAD cyanide (WAD CN); the latter involves both CNL and the weak and moderately strong metallic cyanide complexes, such as cadmium ([Cd(CN)4]2 -), zinc ([Zn(CN)4]3 -, copper cyanides ([Cu(CN)2]-, [Cu(CN)3]2 -, [Cu(CN)4]3 -), nickel cyanides ([Ni(CN)4]2 -) and silver cyanides ([Ag(CN)2]-). CNL includes the CN- ion and the molecular hydrogen cyanide (HCN). In a determined process and depending on the pH value of the aqueous medium with cyanide, the CNT level is always greater than or equal to the WAD CN and the level of the latter should be greater than or equal to the CNL level [1] [2]. According to [8], at an approximate pH of 9.4, the concentrations of CN- and HCN are equal but at a pH of less than 8, almost all of the free cyanide is available as HCN and may volatilize; however, [1] indicates that at a pH of 10 or higher, most of the CNL is present as a CN- ion. For [3], when precipitating iron cyanides, relatively low levels of CNT at pH 7.0 are reached and for [13] and [14] the WAD CN are cyanide species released with a moderate pH of 4.5 and 6 as aqueous HCN and CN- and the majority of the complexes of Cu, Cd, Ni, Zn, Ag.
For the environmental regulations, the maximum permitted limits (LMP) for the emissions with a content of CNT are in the order of 1 mg/L and for the WAD CN in 0.2 mg/L [4]; the United States Environmental Protection Agency (USEPA, by the English acronym) has proposed a regulation for drinking water and aquatic water regarding CNT of 0.2 and 0.05 mg/L, respectively [6] [15] and in terms of CNL for the protection of aquatic life in fresh water is set in 0.022 and 0.0052 mg/L [3]. Peruvian regulations for water quality national standards; establishes the WAD CN in 0.1 mg/L and for CNL is established in 0.022 mg/L.
Usually, the research to degrade the CN- ion is focused on the use of cyanide synthetic solutions to experience its destruction [4] [7] [9] [11] [16] [17]. In [7] when using vacuum ultraviolet and ultraviolet/persulfate UVC/(S2P8)2-there was a complete destruction of 50 mg/L of cyanide in 15 min and 50 min respectively at a pH of 11; [4] when using Caro's acid reduces the initial free cyanide concentration from 400 mg/L to 1 mg/L, after 10 min and at pH of 9 to 11 at 25°C; [9] when evaluating two non-thermal plasma reactors at atmospheric pressure, eliminates 99% of the CNL in both, from an initial concentration of 1 mg/L at a pH of 11, times of 15 and 3 minutes; [11] when reacting a molar ratio of (H2O2 + NaClO)/CN- = 2:1, oxidizes free cyanide by 98%, from an initial concentration of 100 mg/L, achieving a final concentration less than 0.2 mg/L in 20 minutes at a pH of 9°C and 25°C; [17] when increasing the molar ratio [H2O2]O/[CN-]O in the presence of activated carbon impregnated with copper, eliminates more than 90% of free cyanide in 20 minutes at a pH of 11. However, the present study focuses to degrade CNL from the tailings pulp solution, which comes from the treatment of a gold-bearing ore that has been leached using sodium cyanide (NaCN). CNL degradation was carried out using two types of oxidizing reagents in aqueous medium, sodium metabisulfite (Na2S2O5) and the mixture, sodium metabisulfite with hydrogen peroxide (Na2S2O5 + H2O2).
The use of Na2S2O5, in the present study is based on prior grounds of [1] [3] [18], which indicate that Na2S2O5 may act as a substitute of sulfur dioxide SO2 in the INCP (International Nickel Company) process (SO2/air). Although the present study does not replicate the INCP process, but it opens up a new concept in the mixture of oxidizing agents and looks for an alternative to degrade the CNL from the mineral-metallurgical emissions, metallurgists, to apply it industrially. Thus, the aim of the study is to explore a fast and reliable method allowing us to degrade the CNL contained in the bulk of the tailings pulp, coming from cyanidation of a gold-bearing mineral; to comply with environmental regulations.
2. Materials and Methods
2.1 Sample collection
The sample was collected from the ore reduction plant for cyanidation of gold-containing ores of the Machala mining company (El Pro - Ecuador), in moments prior to the tailing evacuation from the stirring tanks CIP (pulp stirring tanks). High density plastic drums were used to transfer the sample to the facilities of the laboratory of the school of metallurgical Engineering at the National University of Trujillo to perform tests.
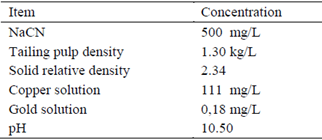
Prior to testing, the contents of the drums were dumped into a high-density plastic cylinder for their respective homogenization and characterization of the sample. 1 liter of pulp was weighed to determine its density (precision balance A&D FX-3000i, 3200 g); a digital pH-meter was used to measure pH (HANNA HI9124N); specific gravity was determined in triplicate using 100 mL volumetric flasks; the copper content was determined by iodometric method, adding KI at 20% and making a titration with sodium thiosulfate in the presence of starch as the indicator; the gold in the solution was measured by atomic absorption (BUCK 210 VGP). NaCN concentration was set at 500 mg/L before each test (Table 1).
It should be noted that the head ore to cyanidation, has as a feature a strong presence of sulphides in the following order: pyrrhotite; pyrite (Fe(1-x)S), chalcopyrite (CuFeS2); arsenopyrite (FeAsS), among other sulphides in minor proportions, such as iron sphalerite (Zn,Fe)S) and galena (PbS).
An essay for the head ore (gravimetric tailings) had the following values: 5 g/t de Au; 18 g/t of Ag, 0.41 % of Cu, 2.45% of As, 14.7% of Fe, 1.89% of Al, 3.59% of Ca, 1.99% of Mg.
2.2 Reagents
For the leaching of the mineral in the processing plant NaCN was used (brand: TAEKWANG IND.CO. LTDA. KOREA, 98%) and lime (P-24, 80% of Ca(OH)2). The reagents used for the tests and analysis were: Na2S2O5 (Diamond Corporation, 97%), H2O2 (TAEKWANG INDUSTRIAL; 1.14 g/mL; 50% weight), AgNO3 crystals (Merck, 4.35 g/mL, 99.8%), KIcrystals (J.T. BAKER, 3.13 g/mL, 100%), NaCl (Merck, 99.5%), K2CrO4 (Riedel-de Haën; 99%) and distilled water.
2.3 Experimental procedure
A four-helix multiple stirrer, of 1 HP at 250 rpm (JUVISA INGENIEROS SAC - Perú) and 4 liter capacity buckets with 2 liters of sample were used for the tests, taken while stirring and measured with a graduated cylinder of 1 liter.
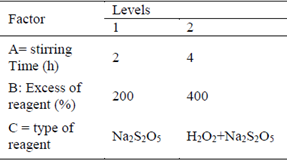
An experimental design of three factors, two levels per factor, considering three replicates per each treatment and CNL degradation as response variable (Table 2).
Upon taking the measurements and making calculations through least squares regressions [3] [5], a two-dimensional sketch was made of the chamber's internal volume (Fig. 4). Then, cross-sections were drawn with the corresponding diameters of the different sections (Fig. 5).
Once the process was finished, a set of surfaces was generated on each of the sections of the geometry; given that these surfaces are closed entities, they generate a solid volume upon ending the operation (Fig. 6) until having a complete reconstruction of the radial geometry of the spiral chamber's internal water volume (Fig. 7).
Tests of degradation by CNL (mg/L), were performed at random and the results (Table 7), were evaluated using the statistical software Minitab 17, with a 5% for the "a" level.
CNL was determined by titration, considering method A from Vogel [19], as well as Standard Methods 4500-CN-D [20]. With the adaptation of a concentration of AgNO3 of 2 g/L (0.01 M) and 1 mL of 5% potassium iodide as an indicator (prevents the overestimation of CNL, in the presence of copper-cyanide species).
AgNO3 was standardized with NaCl 0.01 M, adding 1 mL of 5% potassium chromate (K2CrO4) as an indicator [19-20]; for the titration of the blank a volume of distilled water that was the same as the final volume of the AgNO3 solution (0.01 M) was used, using when titrating 20 mL of the NaCl solution (0.01 M) using as an indicator one mL of the K2CrO4 solution, a correction of 0.1 mL of AgNO3 was deducted. For the calculation of the CNL concentration, the blank was 20 mL of water containing 0.2 mL of a 10 N NaOH solution and 1 mL of 5% KI.
Equation 1 represents the reaction occurred when titrating the cyanidation solution with AgNO3, and serves as a basis to calculate the CNL concentration.
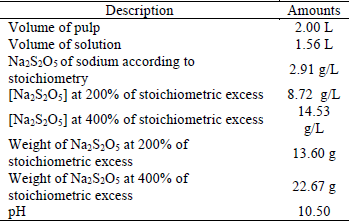
The mass balance for the addition of Na2S2O5 was calculated according to equation 2.
It follows that to neutralize 500 mg/L of NaCN, 2906.65 mg/L of Na2S2O5 are needed; based on this concentration the calculations for the excess % of Na2S2O5 were made to the dosage in the degradation tests of CNL (Table 3).
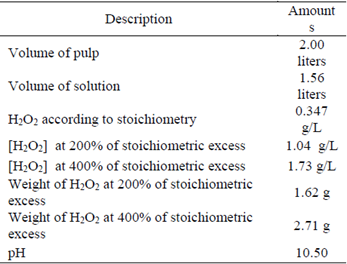
In the case of the addition of H2O2, the stoichiometric reaction according to equation 3 was used [1] [3] [21].
Therefore, to neutralize 500 mg/L of NaCN (265.46 mg/L CN-), 346.87 mg/L of H2O2 are needed; based on the H2O2 stoichiometric concentration and the volume of solution in the pulp, calculations for the H2O2 dosage were made for the CNL degradation tests (Table 4).
The % of degraded CNL was calculated according to equation 4.
Where C i and Cf represent initial and final concentration of CN- (mg/L), for each test, respectively.
3. Results
3.1 Stirring time
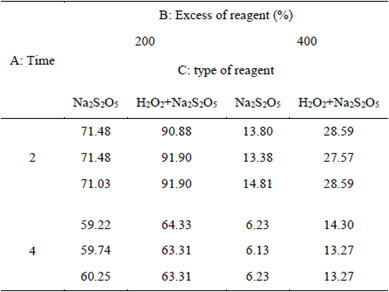
The degradation kinetics of CN- for the factorial design, was tested with 2 and 4 h of stirring time, based on a history of short oxidation times published in several studies [11] [17] [22]. Table 5 shows the results of the residual CNL (mg/L), at room temperature (28°C) and pH 10.5 of original sample stirred at a constant speed of 250 rpm; copper was not added because the original sample had a concentration of 111 mg/L, its presence or addition increases the destruction of the CN- [5] [21] [22] [23].
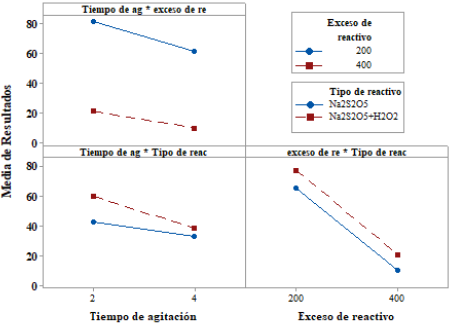
Figure 1 show the degradation of CNL as a function of stirring time, with constant percentages of reagent excess (200% and 400%) and type of reagent (Na2S2O5, Na2S2O5 + H2O2); in addition, it shows the average values of the variation of CNL as a function of stirring time. The results demonstrate that a longer stirring time favors CNL degradation (mg/L) [4] [11] [17].
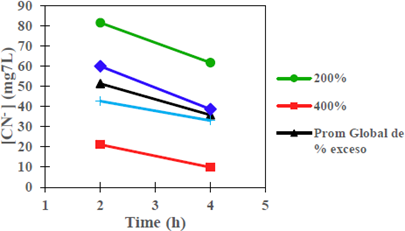
Figure 1 Degradation of CNL as a function of stirring time, with excess % of the reagent and constant type of reagent; at room temperature conditions, [Cu] = 111 mg/L, pH = 10.5 (original sample) at 250 rpm.
It is observed that with 4 h of stirring, the CNL concentration is reduced to an average of 9.90 mg/L, using a reagent excess percentage of 400%. Similarly, it was found that the average variation of CNL as a function of the stirring time is degraded in a higher proportion when Na2S2O5 is constant.
The tendency to converge after 4 h of stirring of the types of reagents, may be explained due to the effect of the reactions of hydrogen peroxide with metallic complexes (coordination compounds) present in the solution from the pulp tailings, there is a release of a significant amount of CN- ion, which delays the degradation of the CNL present in the tailings solution. H2O2 is consumed during these reactions at a high speed and the time will come in which the reagent formed by both compounds will work thereafter as a single reagent (Na2S2Os).
3.2 Percentage of reagent excess
It was found that the CNL degradation is favored with the increase in the percentage of the excess of the reagent [17-22], which is the variable most relevant to the study (figure 4). According to Figure 2, when using a 400% excess of Na2S2O5 reagent, CNL is degraded up to a mean value of 10,1 mg/L, compared to the mixture Na2S2Os + H2O2, with which it was degraded to 20.93 mg/L.
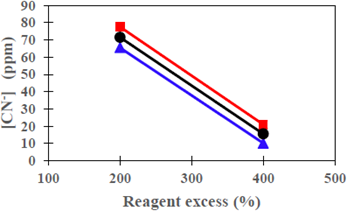
Figure 2 Degradation of CNL as a function of reagent excess (%) with a constant type of reagent at room temperature conditions, [Cu] = 111 mg/L, pH = 10.5 (original sample) at 250 rpm.
Some studies mention that when using H2O2 with the presence of copper in clear solutions, the theoretical consumption is 1.31 g of H2O2 per g of oxidized CN -, but in practice, the consumption is 2 to 8 g of H2O2 per g of oxidized CN- oxidized at a pH between 9 to 9.5 [3] [22] and for the case of the theoretical consumption of SO2 in the INCO process, is 2,46 g/g CN-, but, in practice, the consumption is between 3.5 to 5 g of SO2/g oxidized CN-, using copper as a catalyst and with air injection [3].
In the present study to degrade the CNL of pulp tailings, 6.10 and 10.21 g of H2O2 per g of CN- were added and 27.2 with 45.34 g of Na2S2O5 to 200%, and 400%, respectively; yet, there was a greater degree of oxidation of the CNL. It is assumed that the strong presence of sulfides such as pyrite, pyrrhotite, chalcopyrite, arsenopyrite and galena in the cyanided containing ore, as well as the large amount of metals (iron, copper, zinc, arsenic, etc) present in the solution from the tailings, were strong interfering agents to a greater oxidation of the CNL.
Figure 2 shows clearly the influence of the type of reagent in the degradation of free cyanide when changing from 200% to 400% of their excess (steep slope, in both). There is a slight convergence to 400% excess, so it is expected that increasing the dosage of Na2S2O5 + H2O2; could at some point degrade a similar amount of CNL; the explanation could be taken from [23] when stating that the use of 20 % excess of H2O2 in clear solutions to degrade the cyanide, the reaction rate will increase by approximately 30%.
This reference is taken by not finding records of the use of Na2S2O5 or Na2S2O5 + H2O2; in the degradation of CNL in pulps of cyanided tailings, but it is assumed that the excess mentioned will have to be increased significantly, when making experiments with such means.
3.3 Type of reagent
When assessing the average percentages of CNL degradation, according to the results shown in Table 5, it is determined that when using Na2S2O5, the percentages of CNL degradation are higher than when using the mixture, Na2S2O5 + H2O2. An explanation of this fact could be given according to the last paragraph of point 3.1.
Some authors when they argue that it is preferable to use H2O2 on clear solutions than in pulps, because of its high consumption [1] [3] [21]; however, in the present investigation, we tried to apply it in pulp tailings with high sulfide content, as an adjuvant for the oxidation of cyanide to cyanate, which in turn generates side products such as ammonia (NH3) and carbonate ions (HCO3- ; CO3 2-) [1] [8] [18].
According to the results it can be seen that the reactions of the mixture, Na2S2O5 + H2O2 with cyanide-containing complexes, sulfides, and metals; it had a delaying role in the destruction of CNL, within the limits of the study; this could corroborate the claim of [8] which indicates that, when it comes to sludge with an important presence of sulfides, the peroxide does not adapt well to the system. However, the study tried to elucidate the behavior of Na2S2O5 + H2O2 in degrading CNL contained in a tailings pulp.
To analyze the results, the average, maximum, and minimum mass flow velocities were taken as principal parameters at the outlet of the chambers, in normal manner and on the X, Y, and Z axes to provide validity to the comparison. The results obtained were compared quantitatively amongst them and, likewise, a qualitative comparison was performed of the velocity profiles obtained (Table 2).
Normal velocity values show, qualitatively and quantitatively, that the velocities are in close range, finding slightly higher velocities in the spiral-sectioned chamber. For velocities in the different axes (X, Y, Z), greater differences are present in the velocities obtained; in some cases presenting negative velocities, which would represent zones in the geometry with a high reduction in flow velocity and which the program interprets as negative values.
Nevertheless, some studies have been successful in applying H2O2 as an oxidizing agent for CN-; according to [22] when applying 300 mg/L of H2O2 to tailing pulps with a low sulfide content and in the absence of copper as a catalyst, degrades WAD CN at less than 1 mg/L in 4 h of reaction time. The work in [24] shows that we can effectively eliminate the CN- of synthetic solutions of NaCN, by the generation of H2O2 in situ, using RuO2/Ti as anode and activated carbon fiber as a cathode in the presence of sodium chloride. In another study, [5] manages to efficiently eliminate CN- from a synthetic cyanided solution of sodium cyanide, by oxidation with H2O2 produced with a cathode of copper/activated carbon fiber by an oxidation combined with a Fenton type reaction, and copper oxides generated on the anode surface. The work of [17] degrades quickly CNL of a synthetic solution of potassium cyanide, by oxidation with H2O2 catalyzed with activated carbon impregnated with copper; they found that with an initial molar ratio [H2O2]/[CN-] of 1.5 and 10, more than 90% of the cyanide is removed in 20 minutes.
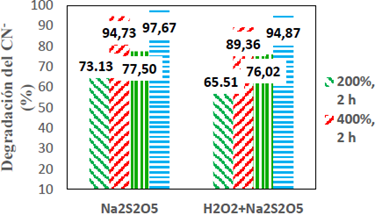
According to Figure 3, when we use Na2S2O5, the highest percentage of CNL degradation was 96.67% and occurred when we used an excess of 400% of Na2S2O5 with 4 h of stirring, compared to 73.13%, which gives 200 % and 2 h of stirring, and for the case of Na2S2O5 + H2O2 with the same parameters of the first case is 94.87% compared to 65.51% with the parameters of the second case. These results indicate that the reagent excess percentage and the time of reaction favor markedly the CNL degradation kinetics. However, when we compared both types of reagents within the framework of the study, we see that when the Na2S2O5 reacts, generates higher percentages of CNL degradation, due to what is detailed in the last paragraph of 3.1.
3.4 Analysis of variance
To test whether the independent variables (stirring time, excess % of the reagent and the type of reagent) have a significant influence on the response variable (CNL), we used the statistical tool ANOVA, determined with the statistical software Minitab 17, with an a level of 5%.
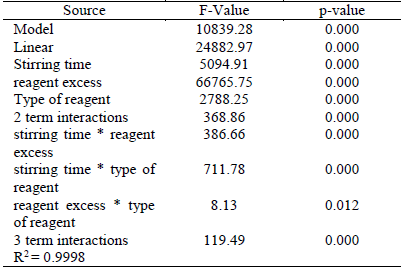
According to Table 6, the factors: agitation time, % of reagent excess, type of reagent and its interactions; it has the p-value, less than 0.05, which shows that the factors influence CNL degradation (mg/L). The regression coefficient of 0.9998%, suggests that the model is significantly high and consistent.
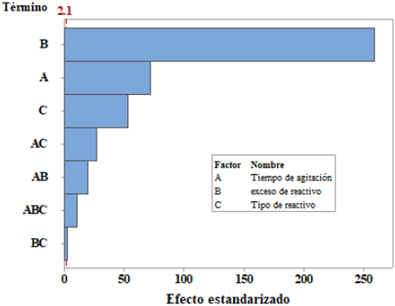
According to Figure 4, the interaction of the stirring time (A) with the type of reagent (C) is the most important of the interactions, both tend to cross within 4 h of agitation, the other interactions are insignificant (straight lines almost parallel); Figure 5 has been corroborated in their order of importance.
In the diagram of Pareto (Figure 5) for standardized effects of design 23, we see that the variables and their interactions are presented in the form of bars staggered according to their order of importance and it shows that the percentage of reagent excess (B) is the most relevant in the CNL degradation, which is confirmed by the F-value in table 6.
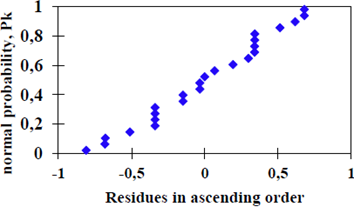
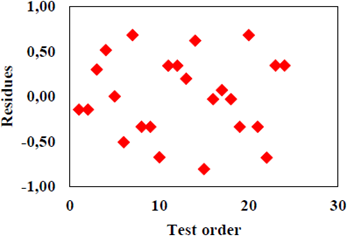
To validate our findings from the analysis of variance we tested the adequacy of the model to the experimental data obtained. According to Figure 6, it is observed that the dispersion of the residuals in ascending order versus normal probability distribution has a linear trend, which confirms the assumption of normality of the model.
Verification for the independence of errors of the experiment results (Figure 7), indicates that the errors are scattered randomly, without an established pattern that could invalidate the experiment.
4. Conclusions
It has been demonstrated that Na2S2O5 manages to degrade CNL in greater proportions than the mixture Na2S2O5+ H2O2 within the limits of the study. The reagent excess percentage, as well as the stirring time, favor a significant degradation of the free cyanide, being the reagent excess percentage the most relevant. A significant percentage of CNL degradation (97.6%) is achieved using Na2S2O5when added to the 400% of their stoichiometric excess with a stirring time of 4h; however, the permissible limits according to the environmental regulations were not achieved.
When analyzing the factorial regression coefficient, the CNL degradation (mg/L) versus stirring time; excess of the reagent and the type of reagent, its value of 0.9998 indicates that the model studied is significant and consistent.
Further research is needed on the theme, as well as evaluation of new conditions closer to the real world, which generate more significant differences in the velocities obtained, or that on the contrary reaffirm the results obtained in this research.
It has been noted that the use of H2O2, on the degradation of the CNL contained in tailings pulps with significant amounts of sulfides, and cyanide metal complexes; is not significant, at least within the concentrations and times studied. However, taking the above into account and the experimental start of a relatively high concentration (500 mg/L of NaCN; 265.46 mg/L of CN-); it is feasible to improve the experimental conditions to increase the percentage of degradation of CNL; increasing a third higher level for the stirring time, or by varying the concentrations of copper and quantifying its final value, a fact that was absent in the present work.