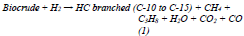1. Introduction
Although the policies of partial replacement of fossil fuels have predominated in the automotive fleet of land transport, the aeronautical sector has not been oblivious to this situation, as it is responsible for 2-3% of CO2 emissions of Greenhouse Gases, GHG, and less than 3% of NOx emissions [1]. Currently, aviation worldwide consumes about 5 million barrels per day of crude, that is, 5.8% of the total world consumption. In terms of conventional fuel, this represents between 1.5 and 1.7 billion barrels per year [2]. The demand for jet fuel is projected to grow 38% from 2008 to 2025, which means an annual growth of 1.9% [1].
Today in the global aviation industry, there are major challenges related to oil prices, national security, environmental impact and sustainability, which make it difficult to have a long-term plan and a budget for operating expenses. In this way, sustainable biofuels produced worldwide offer a solution to these problems. Fuels derived from biomass (biofuels) are a potential alternative to aviation fossil fuel [2]. In recent years, there has been a noticeable growth in the number of researches that seek the partial or total substitution of jet fuel or kerosene for bio jet fuel or biokerosene.
Considering that the electric aircraft is still in the experimentation phase, airlines and aerospace companies believe that biofuels are their best bet to burn less fossil fuels. As discussed above, biokerosene has the same properties as fossil fuel, but with the difference that the former reduces carbon dioxide and total hydrocarbon emissions, due to the significant reduction of aromatics and particulate matter.
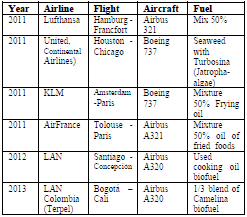
In the search for a more sustainable aeronautical industry, the first cases of commercial flights using biofuels appeared in the year 2011. However, it is worth noting that the initial flights began since 2008. The first commercial flight was carried out by the Virgin Atlantic airline in a B747-400, using a fuel mixture of 20% coconut oil and babassu [2]. The German airline Lufthansa used for the first time a 50% blend of fossil fuel and new generation biofuel to cover the journey between Hamburg and Frankfurt [3]. The airline United, through its subsidiary Continental Airlines, operated its first commercial flight in the United States between Houston and Chicago using a mixture of advanced biofuel derived from algae and traditional turbosina in a Boeing 737 that had already made a demonstration flight using a biofuel obtained from jatropha and algae. Since 2014, they intend to buy 20 million gallons of biofuel per year from Solazyme [4]. Also the airline KLM, on a commercial route between Amsterdam and Paris, used a mixture of 50% conventional kerosene with biofuel obtained from recycled chips oil [5].
Air France made its first commercial flight between Toulouse and Paris using a blend of 50% biofuel from cooking oil used in an Airbus A 321 [6]. In 2012, LAN Airlines and Copec made the first commercial flight in South America using biofuel from cooking oil used in an Airbus A320 between Santiago and Concepción [7] that can see in Table 1.
The first commercial test flight in the country was made in 2013 by LAN Colombia in partnership with Terpel for the Bogotá-Cali route, on an Airbus A320 that used a mixture of two thirds of Jet Fuel A1 with a third of biofuel obtained from camelina oil [8]. Table 1 summarizes the main bio-floats. It is worth mentioning that none of these have used palm oil as a raw material to obtain biokerosene, mainly using Camelina, Algae and fried oil [2].
At the military level, the number of test flights that have been reported has been much lower compared to commercial flights. These have been led mainly by the Navy and the United States Air Force (USAF) since 2010 in an aircraft such as F-22 and helicopters, using mostly 50% mixtures of biofuels obtained basically from algae, camelina or frying oil. Nasa, the Dutch military Air Force, and the European Aeronautics for Defense and Space (EADS) have also participated [2].
According to the director of Boeing Commercial Airplane, Richard Wynne, before generalizing the commercial use of biofuels in the aeronautical sector it is necessary to overcome a series of obstacles [9]:
Fuels will have to be chemically identical and perform the same as existing fuels.
There should be a large enough source for these new fuels to be available at an adequate cost.
Airports must be able to handle fuels without making major changes to storage tanks, pipelines and other infrastructure.
Commercial production must have a price that is favorable compared to conventional fuels.
Fuels must be sustainable, and they should not compete with food sources.
In 2011, it was projected that biofuels for aviation would be marketable in the United States for the transport sector with a mix of 1% in 2015, equivalent to a saving of 16 million gallons of fossil fuels in a year [9]. In 2014, Boeing was interested in using "green diesel" as an option to include it in the jet fuel mixture. In addition, the worldwide industry could supply 300 million gallons per year to cover 1% of the demand for fuel for airplanes. Boeing and the 27 airlines of the Sustainable Aviation Fuel Users Group are committed to develop a biofuel produced in a sustainable manner, without negative impact on GHG, local food security, soil, water and air [10].
In 2016, it was estimated that biorefineries worldwide could supply up to 100 million gallons per year, which is still unmatched by the 83,000 million gallons of fossil fuel that air companies consume annually [11].
In the United States, Syntroleum formed a joint venture with Tyson Foods to produce renewable diesel and aviation fuel using the Biofining process. Synthetic fuel production in its first plant was approximately 5,000 barrels per day in 2010. The North American AltAir fuel plant in Paramount, which started in 2015, produces 2500 bbl/day of aviation renewable fuels using non-edible fats and oils from agricultural waste (Lane, 2016). The "Biofuel Flight Path", which is an initiative of the European Commission, aims to produce 2 million tons of biofuel in Europe by 2020 [12].
In 2009, the International Air Transport Association (IATA) announced a commitment in three steps for the aviation industry to achieve growth with carbon neutral. On average, it seeks an improvement of 1.5% per year in fuel efficiency from 2010 to 2020; growth in carbon neutral since 2020; and 50% reduction in carbon emissions in 2050 compared to the 2005 level [1].
Currently, aircraft with jet engines, both civilian and state, tend to use fossil fuels derived from petroleum, which are not renewable and are extremely polluting. In the global tendency to look for new alternatives, the Colombian Air Force (FAC) is not the exception. However, the country does not have the capacity to perform the functional tests required of these biofuels, since there are no power plants or test benches for this purpose.
This type of components is required for the validation and correlation of yields between traditional fuels and biokerosene.
In addition, the issue of fuels in the FAC is critical, since it represents between 60 and 65% of the budget. Analyzing the period between 2010 and 2016, in 2013 there was a record expenditure of $ 38.55 billion corresponding to the consumption of 404.55 thousand barrels of fuel. The annual consumption of fuel by the FAC has had a notable decrease since 2013, possibly due to the post-conflict effect.
Today the FAC counts with an aircraft that uses Turbofan engines with a high derivation index for approximately 12 years. The aircraft is used as an aerial intelligence platform, gathering information that allows strategic decision-making for the defense of the country and the fulfillment of the mission of the FAC. Others are used for VIP transport, according to the institutional and national government requirements.
In addition, SATENA has aircraft used for passenger transport and is the only airline that fulfill itineraries in some parts of the national territory that are fundamental for the economic development of the regions of the country. Due to institutional policies, a continuous airworthiness and an operational readiness of 100% of these aircraft is required. For this reason, the minimum scheduled maintenance time is required in three levels, with level 1 and 2 being carried out in Colombia by the FAC.
At present, the FAC does not have the ability to perform level 3, thus it is done outside the country, increasing costs, affecting the safety and reliability result. Therefore, it is necessary to develop a test bench that allows to perform and verify the correct operation of the engines of this type of aircraft and simulate unforeseen operating conditions. As starting point, there are two high-cost turbines that have variable measurement systems associated with power and emissions.
The research proposed in this work aims to study the production and/or use of an alternative fuel, biofuel, renewable and as sustainable as possible, for the diversification of the national energy basket, especially in the air transport sector. This fuel can be biokerosene or special FAME for aviation, which are intended to be used in mixtures with Jet Fuel in a turbine test bench.
The medium term considers the possible implementation at the industrial level of a process for obtaining aviation biofuels by different routes, which will be generally described below. The use of biomass as main raw material in these processes implies greater technical and economic efforts.
This requires studies focused on the research of improved catalysts, the exploration of alternative sources of biomass and other available and profitable reagents, the detailed determination of the kinetics and the optimization of the reaction conditions.
2. Production of Jet Biofuels
The production of biofuels for aviation can be given by different technologies, which can be differentiated in terms of the flexibility of raw materials, the degree of development and implementation, the product quality obtained, and investment in the production plant. The most developed technology until now is the production of biokerosene by hydroprocessing. However, obtaining FAME biodiesel by transesterification can also be considered, as it can be mixed with Jet Fuel. Other interesting but less balanced processes in the aforementioned aspects are hydroxyalkylation, pyrolysis, cracking catalytic, Fisher-Tropsch synthesis, among others.
2.1 Hydroprocessing
As oils from biomass treatments can contain a large proportion of oxygen, which is undesirable in transportation fuels, it is necessary to remove these important amounts of oxygen. This process is known as deoxygenation [13]. In a simple way, hydro processing is the conversion of a substrate made by hydrogen, thus it can also be known as hydro conversion.
The hydroprocessing of triglycerides to obtain biokerosene is the most well-known and industrially used way, thus many alternatives have been studied to increase the stability of the catalyst and avoid leaching. In contrast, research on other routes, such as pyrolysis and catalytic rupture, is limited. As a result, operational difficulties have not been overcome and these technologies are still not applied at large scale.
To obtain cleaner fossil fuels for transport and energy generation, the hydrogenation of crude fractions is carried out using catalysts, generally of metallic and acid character (bifunctional), to remove sulfur, nitrogen and/or unwanted oxygen from the cuts.
Since the 1980s, researches have look for the way to extend the same process to biomass as a raw material and the possibility of removing oxygen from oil structures as CO2 using atmospheric fluidized bed catalysis (FCC) or hydrotreating to high pressures, although oxygen also is removed in CO and H2O way, CO2 is less dangerous. However, the results obtained showed very low efficiencies of about 20%, mainly due to the carbonization of the material.
Current research indicates that the adequate conditions for hydrotreating are between 300 and 400 °C and residence times greater than 1 hour, as well as the presence of water from the start to avoid carbonization and the use of high pressures [14]. Deoxygenation by hydroprocessing consists of a large number of reactions that can be grouped either as hydrocracking or as hydrotreatment [15].
In hydrocracking as a hydrogenolysis, hydrogen plays a more destructive role, fragmenting large molecules into smaller ones by breaking the carbon-carbon bonds and isomerization. To achieve this and reduce undesired side reactions, such as polymerization leading to coke formation, high temperature and pressure conditions are used, also this condition is important for increase selectivity and efficiency of catalyst because According to the morphological and chemical properties of the catalyst, the selectivity of the process can be varied [16]. Additionally, in hydrotreatment the role of hydrogen is not so destructive, since the aim is to remove unwanted elements such as sulfur, nitrogen and oxygen, which are removed as hydrogen sulfide, ammonia and water, respectively, without altering significantly the length of the molecular structures. To reduce the indiscriminate breaking of bonds and promote selective breaking, the operating conditions are less intense than in "hydrocracking". Traditionally, in the petrochemical industry bimetallic catalysts, such as NiMo, CoMo or NiW/y-AbO3 there are supported in aluminosilicates and alumina for the hydrotreatment of medium and heavy fractions in the refining of crude oil; given its high hydrogenating activity, its use has been extended to the hydrotreatment of acylglycerides to obtain biocheresin [17].
The main objective of the bio-oil hydrotreatment to obtain biokerenes is the production of branched hydrocarbons saturated with 14 to 20 carbons. The most desirable are C15 to C18 iso-alkanes, which represent a liquid mixture of hydrocarbons with a boiling range similar to that of kerosene [15]. However, hydrotreating a biocrude involves a complex set of reactions. It begins with the hydrogenation of triglyceride unsaturations, then the hydrogenolysis of these compounds takes place and propane and fatty acids are obtained, which can be decarbonylated, decarboxylated or hydrodeoxygenated.
This implies the removal of oxygen and the production of n-alkanes. In the decarbonylation (DCN) and decarboxylation (DCX) reactions, oxygen is released in the form of water and carbon monoxide or dioxide, respectively, so that aliphatic chains with one carbon less than the original fatty acid chain are obtained [18]. Meanwhile, in the hydrodeoxigenation (HDO) reaction, the oxygen is removed as water only, thus the carbon skeleton remains intact.
The fuel produced in these reactions is subsequently subjected to processes of isomerization, that is a process where a molecular reorganization occurs to obtain branched iso-aliphatic chains of about 10 and 15 carbon atoms that possess the characteristics of the desired fuel, biokerosene.
According to the above, all reactions could be summarized in one to describe the global conversion of the biocrude during hydrotreating (See Equation 1):
Considering the concept of atom economy, the most desired route in the hydrotreatment of triglycerides is the HDO on the DCX/DCN. as already argued in previous paragraphs. In any case, although it is not possible to prevent the elimination of oxygen in the form of carbon oxides generated by the alternate DCX/DCN routes, it can be minimized by prioritizing the HDO. This is achieved with the nature of the catalysts and the conditions of operation: high partial pressure of hydrogen and temperatures between 200 to 400 °C [19].
Furthermore, it is necessary to take into account that once carbon dioxide is formed by DCX and carbon monoxide by DCN, the reactions of methanation and water gas shift reaction are presented. This can modify the conversion and thus the selectivity of HDO [20]. Intensive pressure and temperature conditions are not the only unfavorable aspects of hydrotreating, but there is also a significant consumption of hydrogen.
The technology of the Honeywell company UOP LLC was selected by Petrixo Oil & Fas to produce renewable aviation fuel (renewable jet fuel) and renewable diesel in a new refinery in Fujairah, in the United Arab Emirates. The refinery can process up to 500 thousand t of a large variety of renewable raw materials. In 2014, it achieved a design capacity of 1 million tons of biofuels per year with an investment of US$ 800 million, becoming the first commercial scale production of jet fuel outside of North America.
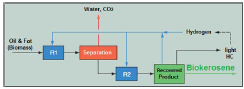
This technology at commercial scale is better known as the Ecofining™ process (UOP brand LLC and Eni sPa), in which two stages of the hydrorefining process are integrated, as shown in Figure 1.
The feed is driven to the process pressure, mixed with recycled hydrogen (from a multi-stage adiabatic separator), then sent to an HDO catalytic reactor (R1), where the oil biomass is completely saturated and deoxygenated. The gas recycle to R1 is configured, so that the partial pressure of hydrogen is minimal at the output of the reactor. The conversion of the feed is complete, the yield of deoxygenation to produce the hydrocarbons is very high (8090%), as well as the selectivity to paraffins with the boiling range of kerosene. The primary reaction of deoxygenation has propane, water and carbon dioxide as by-products.
The effluent of R1 is immediately separated at reactor pressure to remove carbon dioxide, water and low molecular weight hydrocarbons. The resulting diesel with excess hydrogen is taken to the hydroisomerization reactor (R2), where the biokerosene formed by branched alkanes is produced. The removal of excess hydrogen for the isomerized product is done in a conventional gas-liquid separator.
After purification, the hydrogen is recycled to R1. Hydrogen is added to the process to balance both chemical consumption and replenish losses. The liquid product obtained is sent to a recovery section that uses conventional distillation to separate co-products, such as propane and naphtha [21]. More details about this process have been published by Baldiraghi et al. (2009) [22] and Lavrenov et al. (2011) [23].
Deoxygenation and isomerization have been integrated in a single step to produce a non-ester biodiesel from soybean oil, called isodiesel, which contains not only branched alkanes, but also naphthenes (cycloalkanes) and monoaromatics. The latter affects the density and the cloud point. Using the Pt/SAPO-11-Al2O3 catalyst in the presence of hydrogen (phosphoaluminosilicate zeolite), in the one-step reaction mechanism, it was observed that DCX/DCN takes precedence over HDO [24].
In general terms, the main challenges for the hydroprocessing of biomass to generate high quality biofuels in a sustainable way are: flexibility in the feed in, reactor configurations that combine the reaction and separation steps, improvement of catalysts to increase stability and reduce pressures of hydrogen operation; hydrogen handling; and general energy efficiency related to the integration of processes (energy and mass), waste management, scaling, etc. [25].
2.2 Co-processing
In hydrogenation by co-processing, the vegetable oil is mixed with the mineral diesel (pre-treated or not) for further treatment with hydrogen in the plants located in conventional oil refineries, in order to produce a single fuel outlet combined [23]. Some studies have been made on co-processing of waste mixtures of soybean oil with natural gas to obtain transportation fuels [26]. Since 2000, research has been carried out that has shown to have high efficiencies in fluidized bed catalytic processes [14].
For this last option, it is worth mentioning that, in the strategic profile of Ecopetrol for 2020, co-processing is in the strategy as a post-transesterification and fermentation process, rather than projects for other second-generation processes, since a large number of oil companies are making large investments in R & D + i for advanced biofuels (high quality). For this reason, the Colombian State is monitoring and developing new processes such as Biocetane (Green Diesel) and Biojet, which are in stages of regulation and technical validation for industrial scaling.
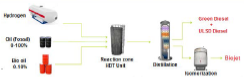
Regarding biojet, in Ecopetrol the process is in phase of monitoring and development of new processes, where a market analysis has been established, a pre-feasibility study has been carried out and the potential for biojet production has been determined by co-processing in the refinery. Some approaches have been made with airlines to join efforts in the biojet researchbiojet [27]. Figure 2 shows a co-processing process for obtaining biojet, where a hydrotreatment with hydrogen gas from a mixture of diesel and biocrude is carried out with the subsequent distillation followed by isomerisation of the bottoms to obtain the fuel for aviation.
2.3 Hydroxyalkylation
Another alternative process to oil hydrotreatment is the hydroxyalkylation of aromatic fuels that allows to obtain fuel oil used in aviation and has the great advantage of working with lignocellulosic biomass. In this case, the use of protonated titanium nanotubes (PTN) has good properties for the hydroxyalkylation/alkylation of 2-methylfuran and n-butanal from lignocellulose, thus it has been researched.
Subsequently, the product of this process was HDO on nickel supported on zeolite to obtain branched alkanes, base of the aviation fuel, showing that the Ni/H-ZSM-5 is the catalyst with the best catalytic behavior and great stability, which makes it promising for future applications [28].
Since 95% is of fossil origin, an option to feed hydrogen gas for hydrotreating is the application of hydrogen donors that, in addition to providing it, offer an excellent capacity for the stabilization of fuels, such as those of aviation.
In these fuels, 1,2,3,4-tetrahydroquinoline (THQ), 1,2,3,4-tetrahydronaphthalene, and trans-decahydronaphthalene, generally aromatic with condensed nuclei have been used. Its action lies in limiting the formation of aliphatic radicals by temperatures above 300 °C, in order to produce mainly C2 and C3 alkyl aromatic compounds.
In this stabilization, concentrations up to 5% of mass of these hydrogen donors are used. Some references refer to antioxidants added as additives in a range of 50 to 500 ppm by mass, but for performance and economic reasons, it is desirable to reduce these concentrations as much as possible. These results have been applied for the stabilization of the FAME at high temperatures [29].
However, this alternative as hydrogen source is still very expensive compared to the production of hydrogen from the electrolysis of water, or by reforming methane.
2.4 Catalytic and Thermal Cracking
Regarding oil fractions, the yield towards the desired products in the catalytic rupture of residual biomass can be increased by co-feeding hydrogen donors such as methanol, tetralin and decalin, which allows to reduce the undesirable deposition of carbon on the catalyst that generally leads to inactivation by the formation of coke.
These donors not only promote rupture and hydrogenation reactions, but also serve to dilute the lignin, inhibit the polymerization reactions that can occur at high temperatures, and contribute to the reduction of the volatility of the obtained in the biocrude. In this sense, the formation of carbon and coke deposits in fixed-bed reactors during the pyrolysis of wood can be reduced to produce bio-oils, as a result of low H/C ratios and the phenolic nature of [30].
For the hydroprocessing of bio-oils generated by the pyrolysis of residual lignocellulosic biomass, there is a complex study, related to the type of reactor, catalyst, temperature and pressure, spatial velocity, selectivity and yield, for different types of substrates such as wood, rice husk and sawdust [31].
An interesting process where vanillin (from lignin) is taken as a model molecule on a catalyst composed of Pd nanoparticles supported on TiO2 and modified nitrogen on porous carbon (Pd/TiO2 @ NC) uses formic acid as a hydrogen source to perform deoxygenation [32].
2.5 Other processes
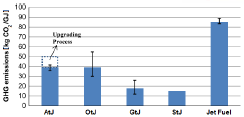
In addition to the hydrotreating route of bio-oils to obtain biojet (OtJ), there are other options such as Alcohol to Jet (AtJ), Gas to Jet (GtJ) and Sugar to Jet (StJ), which also change the starting biomass as raw material. A comparison in terms of GHG emissions from each of these routes with respect to fossil kerosene can be observed in Figure 3[2].
3. Characterization of Aviation Biofuels
Regarding the characterization and compliance with the quality specifications of aviation fuel, we have the ASTM D7566-16b (Standard Specification for Aviation Turbine Fuel Containing Synthesized Hydrocarbons) and NTC 1899 (Petroleum and its Derivatives, Turbo Fuel for Aviation). For the Jet Fuel A-1, there are the ASTM D1655-04, IATA, Def Stan 91-91 and ASTM D7566. The previous norms contain basically the limits for the specifications and characteristics of the fuel. Nevertheless, a set of norms for the methods and tests required to determine each of the specifications related. The main specifications are about composition (total acidity, aromatic content and total sulphur), volatility (distillation curve, flash point and density), fluidity (freezing point and viscosity), combustion (lower calorific value, smoke point and content of naphthalenes), thermal stability (JFTOT, Deposits), conductivity and lubricity.
4. Methodology
In the country the only precedents of the use of biofuels in aviation in Colombia are the LAN flight of 2013, and some biojet fuel production trials carried out by Ecopetrol during those years, through the ICP.
4.1 Proposal
The project FAC-ECCI, called "Use of biofuels in aircraft of Colombian Air Force", which is financed by COLCIENCIAS, intends to evaluate the use of fuel mixtures with biofuel (biokerosene and FAME) for the determination of their behavior in aviation turbines for their future implementation in aircraft of the FAC. This requires to:
Establish the biofuel production process at the laboratory level for the evaluation and comparison of the physico-chemical properties of the fuel mixtures used in the turbine tests.
Adapt the test bench with emphasis on the parameters of metrology, civil works and acquisition of data necessary for its operation to develop runs with fuel mixtures.
Analyze the behavior of the fuel mixtures in the turbines, in terms of emissions and power, comparing with the results of the simulation used as support.
Evaluate the sustainability of the biofuel used through the analysis of the life cycle and value chain, as well as carbon footprint.
4.2 Application
With regard to the production and use of biofuels in the two turbines, the following methodological aspects have been defined.
In this way, it was proposed initially to use mixtures of biokerosene with Jet Fuel in the PT6 turbohelix engine of the FAC at the CAMAN base (Madrid, Colombia). The production at the pilot plant level of this biofuel was explored, but the cost of the heterogeneous reactor at this scale was beyond the scope of the project. Besides, there is no trickled bed reactor in the country that produced at least 1000 gallon per year. However, biokerosene production was explored at the laboratory scale as indicated below.
As not enough biofuel was available to make mixtures for tests in engine banks, the use of biodiesel FAME mixed with jet fuel was discussed, based on previous research that suggested that they could test up to 20% mixtures [33], [34], [35], [36]. It was analysed that short chain methyl esters would be sought so that the FAME biodiesel not significantly affected the cold properties of the Jet Fuel, which implied the transesterification of oils such as the palm kernel.
In this way, a study of the production of biodiesel FAME from palm kernel oil at laboratory scale was carried out. In this transesterification, the temperature, the amount of catalyst (% KOH) and the methanol-oil ratio were varied to study the effect on the production yield of methyl esters.
With the value found for the optimal process condition in the production of palm kernel oil FAME at laboratory scale, the kinetics were performed and the production was scaled at bank level.
Mixtures of 10%, 20%, 30%, 40% and 50% between FAME palm oil (biodiesel 360 provided by BioD S.A.) and Jet Fuel A1 (donated by Terpel) were used in the turbojet engine test bench J69 of the Colombian Air Force (FAC). For the test bench of the PT6 turbo-helix engine, mixtures of 5%, 10%, 20% and 30% will be tested. To analyse the possibility of carrying out air tests with mixtures of FAME biodiesel with
Jet Fuel in an aircraft of the FAC, it is necessary to evaluate cold properties such as the cloud point and the freezing point. In this way, the improvement of the cold flow properties of these mixtures using additives is contemplated.
At the same time, biokerosene was obtained at laboratory scale by hydrotreatment employing 1% Pt catalyst and several types of zeolites (USY, H-p, ZSM-5 provided by Zeolyst International), three types of raw materials (soybean oil, palm oil and palm kernel oil), two temperature levels and two levels of reducing atmosphere concentration. The main purpose was to develop the process in one step.
5. Advances and Preliminary Results
The details of each of the results obtained are presented in specific publications. However, the following is a general presentation of the advances that we believe may contribute to the state of the art presented in this work. The results obtained at the laboratory level of FAME from palm kernel oil and biokerosene are presented below.
5.1 FAME
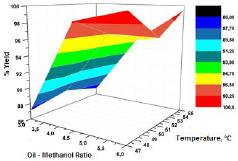
The results obtained based on the application of the Box-Behnken design showed yields of biodiesel production in the range of 85% and 99.9%. Figure 4 shows the effect of the temperature and molar ratio on the biodiesel yield.
The increase in the amount of methanol favors the yield when the amount of catalyst does not exceed 1% per weight of oil. However, it is necessary to guarantee an effective separation process of sodium methoxide from biodiesel for refining.
The best performance occurs when low catalyst concentrations and low temperatures are used, guaranteeing a yield higher than 98%. The catalyst concentration was correlated with the molar ratio of methanol-oil, where the low concentration of KOH and the high molar ratio provide an optimum performance in the production of biodiesel from crude palm kernel oil.
Consequently, the operating conditions were established to reach the production of biodiesel at laboratory scale:
Load: 27.5% of methanol and 0.5-1% of KOH regarding oil in mass.
Conditions: 47 °C, 500-750 rpm, 1.5 hours.
Based on the characterization tests developed, the biodiesel produced from palm kernel oil at laboratory scale is within the parameters established by the American and European standard.
According to the results of cloud point, it is recommended to deepen the research on the use of additives that reduce the temperature of crystal formation in biodiesel. It is recommended to design a reactor that increases the surface contact between the reactants in order to guarantee the highest conversion of fatty acids to methyl esters. It is also recommended to standardize a separation method that involves a minimum energy consumption.
5.2 Biokerosene
The experimental work conditions are 250 °C, cold initial pressure of 10 bar with a composition of 70% nitrogen and 30% hydrogen, magnetic stirring of 500 rpm and upon reaching the working temperature of reaction for 6 hours, taking as reference several previous studies [37], [38].
The preparation of the sample is 16 grams of the used oil, the catalyst is 10% per weight of the amount of oil with 1.6 grams.
It was defined that of these 1.6 grams, 0.125 grams are of the impregnated catalyst and the rest of the catalyst without impregnation. To complete the necessary volume in the reactor, 64 grams of hexane are added as solvent.
Due to the limited amounts of the catalysts synthesized, the following experimental design was proposed. The first stage of selection consists of reacting with each oil and each catalyst to the same working conditions. This resulted in 6 reactions.
Then, for choosing the best oil and catalyst, working conditions are changed: first at 300 °C with the same composition of the nitrogen/hydrogen atmosphere and in the final stage the temperature that best results and the composition of the nitrogen/hydrogen atmosphere at 30%/70%.
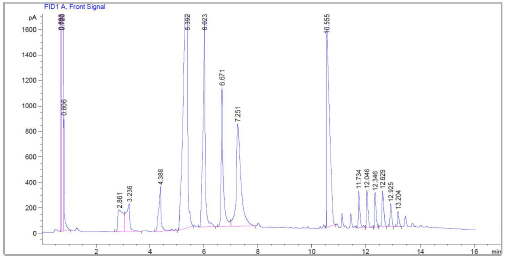
The greater amount of n-dodecane was produced using palm kernel oil, platinum with zeolite H-p, at 300 °C and with an atmosphere of 30% hydrogen/70% nitrogen. This can be seen in the chromatogram of the Figure 5, where the largest peak for hydrocarbons generated from C10 to C18 corresponds to the n-dodecane, alkane that represents aviation fuel.
The first peak corresponds to the solvent, n-hexane, while the peak of the internal standard is at 10.5 minutes, before the triglyceride peaks that did not react.
6. Conclusions
Despite the interest of the aeronautical industry in incorporating biofuels that contribute to the reduction of greenhouse gases, it is still necessary to develop further research to ensure that technological processes reach a commercial level attractive to the sector.
Currently, the main processes for obtaining biofuels for aviation are transesterification and hydrotreating. The first is a transesterification process that operates under moderate conditions, thus operating costs are low despite the fact that the product (biodiesel FAME) has quality limitations that restrict the mixtures with the fossil fuels for aviation.
Mainly power calorific and cold properties are affected. In contrast, hydrotreating achieves a product (biokerosene) with an equal or superior quality to that of conventional Jet Fuel, that is, without limitations in the mixture. However, it requires a catalytic process that manages hydrogen gas at high pressure, which makes it at the moment very expensive and unattractive.
FAME from palm kernel oil mixed with Jet Fuel tends to have better cold properties due to the shorter chains of the methyl esters obtained from palm oil. While, in obtaining biokerosene at the laboratory level, also from palm kernel oil, it was shown that it is possible to obtain characteristic hydrocarbons in a single step using a mixture of catalysts.
Both processes are still under study to improve the yield towards the products of interest. Hydroprocessing to obtain biofuels has been studied with great intensity in the last eight years, using a wide variety of vegetable oils, catalysts, reactors, and, especially, different process conditions. The greater quantity and diversity in the works is in the aspect of catalysts, which has provide a complete classification of catalysts according to their reactivity and/or selectivity for this process, in addition to allowing hydrogenation and isomerization in a single stage.
The main challenges that research must face for biomass hydrotreatment processes to produce high-quality sustainable biofuels are: flexibility in the feed in terms of composition and unwanted impurities, reactor configurations that combine the reaction and separation steps, improvement of catalysts to increase stability and reduce pressures of hydrogen operation; hydrogen handling; and general energy efficiency related to the integration of processes (energy and mass), waste management, scaling, etc. There is also the need to develop more studies that minimize the unnecessary consumption of hydrogen during hydrotreatment, mainly due to secondary reactions. Although this technology is expensive, the reuse of CO2 an H2O can be a value that reduces the high costs.