Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista Ingeniería Biomédica
versión impresa ISSN 1909-9762
Rev. ing. biomed. vol.2 no.3 Medellín ene./jun. 2008
ISOLATION OF HUMAN BONE MARROW MESENCHYMAL STEM CELLS AND EVALUATION OF THEIR OSTEOGENIC POTENTIAL
Felipe García Quiroz1,2,5, Olga M. Posada Estefan1,2, Daniel Gallego Pérez2,3, Natalia Higuita Castro2,3, Carlos A. Sarassa Velásquez2, Derek J. Hansford2, Piedad Agudelo Florez4, Luis E. López Rojas1,2
1 Programa de Ingeniería Biomédica EIA-CES. Línea de Biotecnología en Salud y Biomateriales, Colombia.
2 Grupo de Investigación en Ingeniería Biomédica EIA-CES (GIBEC).
3 Biomedical Engineering Department, The Ohio State University, E.E.U.U.
4 Investigadora, Instituto Colombiano de Medicina Tropical (ICMT), Colombia.
5 Contact e-mail: bmfegar@eia.edu.co.
ABSTRACT
Human bone marrow mesenchymal stem cells (hBMSCs) comprise a cell population capable of self-renewal and multilineage differentiation commonly isolated from bone marrow aspirates of large bones. Their osteogenic potential has been extensively exploited for the biological evaluation of scaffolds or biomaterials with applications in bone tissue engineering. This work aimed to isolate hBMSCs from femoral heads of patients undergoing total hip arthroplasty and to evaluate their osteogenic potential. Briefly, the trabecular bone was extracted and mechanically disaggregated; the released cells were cultured and non-adherent cells were removed after 4 days. The osteogenic potential was evaluated at the fifth passage after 14 and 20 days of induction, comparing cultures with and without osteogenic supplements, via Alizarin red staining and the quantification of the gene expression levels of the osteogenic markers collagen type I, osteonectin and bone sialoprotein through real-time RT-PCR. The obtained hBMSCs presented a stable undifferentiated phenotype after prolonged cell culture, matrix mineralization capabilities and expression of osteoblast phenotype upon osteogenic induction. The three markers were up-regulated in cultures under osteogenic conditions and 2 fold differences in their expression levels were found to be significant for the onset of the differentiation process. The obtained hBMSCs may have applications on the in vitro evaluation of the osteoinductivity of different biomaterials, bioactive molecules or tissue engineering scaffolds.
KEY WORDS: Cell differentiation, Mesenchymal stem cells, Mineralization, Osteogenic markers, Tissue engineering.
RESUMEN
Las células madre mesenquimatosas de médula ósea humana (abreviadas hBMSCs) constituyen una fuente de células auto-renovables con alto potencial de diferenciación, comúnmente aisladas a partir de los aspirados medulares en huesos largos. Su diferenciación hacia el linaje osteogénico, por ejemplo, ha sido ampliamente utilizada para la evaluación biológica de biomateriales o matrices con aplicaciones en la ingeniería de tejidos óseos. El objetivo de este trabajo consistió en aislar hBMSCs a partir de la cabeza femoral de pacientes sometidos a artroplastia total de cadera, así como evaluar su potencial osteogénico. Brevemente, se extrajo el hueso esponjoso y se disgregó mecánicamente; las células desprendidas se cultivaron y las células no adherentes se eliminaron luego de 4 días. El potencial osteogénico se evaluó en la quinta generación de cultivo, mediante ensayos de diferenciación a 14 y 20 días donde se compararon cultivos con y sin suplementos osteogénicos. La evaluación se realizó mediante tinción con Alizarina Roja y la cuantificación de los niveles de expresión génica de los marcadores osteogénicos colágeno tipo I, osteonectinca y sialoprotiena ósea mediante RT-PCR en tiempo real. Las hBMSCs obtenidas presentaron un fenotipo no-diferenciado estable, así como la capacidad de mineralizar la matriz extracelular y expresar un fenotipo similar al osteoblasto durante la inducción osteogénica. Los tres marcadores evaluados se sobre-expresaron en los cultivos en condiciones osteogénicas, y se encontró que cambios hasta de 2X en sus niveles de expresión son relevantes para el desarrollo del proceso de diferenciación. El modelo de hBMSCS presentado podría ser utilizado para la evaluación in vitro de la osteoinductividad de diferentes biomateriales, moléculas bioactivas o matrices para ingeniería de tejidos.
PALABRAS CLAVE: Diferenciación celular, Células madre mesenquimatosas, Mineralización, Marcadores osteogénicos, Ingeniería de tejidos.
I. INTRODUCTION
Mesenchymal stem cells obtained from human bone marrow (hBMSCs) have been widely studied because of their relative easy access and differentiation potential to the osteogenic, adipogenic and chondrogenic lineages, and other kind of tissues or cells, including hepatocytes, cardiomyocytes and neurons [1-2]. Their multipotentiality and self-renewal has increased the attention to this stem cell model as a self-renewing cell source with applications in tissue engineering and regenerative medicine [3-4]. Particularly, hBMSCs osteogenic potential has been extensively explored in the biological evaluation of bone tissue engineering scaffolding structures [5-7]. In addition, their isolation based on the adherence to the culture substrates constitutes a straightforward strategy for elimination of non-mensenchymal lineages, reducing the dependency on complex cell isolation methods which rely on the expression of specific surface markers [1, 8].
Although autologous transplantation of in vitro expanded stem cells, via direct cell implantation on the site of the lesion or using cell-loaded engineered scaffolds, holds great promise, the common strategies for cell harvesting require somehow traumatic procedures for aspirating the bone marrow [1, 6]. Therefore, in those studies where an autologous model is not required, it would be advantageous to work with a mesenchymal cell line displaying similar characteristics that could be isolated without inducing any trauma in the donor site or from tissues otherwise discarded in conventional surgical procedures. One example of such studies is the initial development and evaluation of new biomaterials or scaffolds, which may be tested and optimized in vitro prior to the in vivo studies that would require an autologous cell source [9-11]. Therefore, using a non-autologous model would help to increase the availability of biological samples (e.g. donors) for experimentation as well as reduce the costs of these studies.
This work presents a method for the isolation of hBMSCs via processing of bone marrow and trabecular bone intermixtures from human femoral heads of patients undergoing total hip arthroplasty, and the subsequent characterization of their osteogenic potential by the assessment of calcium deposition on the extracellular matrix and the evaluation of the gene expression levels of osteogenic makers known to actively participate on the differentiation/mineralization process.
II. MATERIALS AND METHODS
2.1 Human bone marrow mesenchymal stem cells isolation and culture
hBMSCs populations were extracted by the processing of the femoral head of three patients undergoing total hip arthroplasty, following a protocol previously described by the authors with some modifications [12]. The extraction protocol was approved by the Committees for Ethical Issues of the CES University and the Hospital Pablo Tobón Uribe and all samples were processed after written informed consent was obtained. The biological material used in this study would have been otherwise discarded during the standard surgical procedure. Briefly, under the sterile conditions of the operating room the femoral heads were segmented transversally into two hemispheres to expose the trabecular bone, which was later extracted with successive washes with phosphate buffered saline solution (PBS) (Gibco, USA) to facilitate the disaggregation of the tissue, and mechanically dissected to obtain fragments of approximately 2 mm3. The obtained solution from each hemisphere was recollected and filtered with a 70 µm cell strainer (Falcon, USA) before centrifuging at 400 g for 10 min. Cell pellets were resuspended in non-osteogenic media (NO) consisting of Dulbecco's modified Eagle's Medium (DMEM) (Sigma, USA), supplemented with 10% Fetal Bovine Serum (FBS) (Gibco, USA) and 1% Antibiotics (streptomycin and penicillin) (Gibco, USA), and cultured in 25 cm2 flasks at 37 °C in a humidified atmosphere containing 5% CO2. At day 4, the cultures were washed with PBS to remove the non-adherent cells and further expanded until ~ 80% confluence, when they were harvested and expanded in 75 cm2 flasks. After subculture, these cells were designated as passage 1. In summary, the new protocol comprised two modifications: a reduction of the seeding area for the primary culture, with 50 cm2 for each hemisphere, and removal of the non-adherent cells 4 days earlier in the cell culture history. The extracted hBMSCs were evaluated in terms of cell morphology and mineralization capability (qualitatively) but only cells from one donor at passage 5 were used to study the gene expression patterns of osteogenic markers.
2.2 Differentiation to the osteogenic lineage
Osteogenic differentiation was evaluated at 14 and 20 days post induction via Alizarin Red staining and quantitative RT-PCR (RT-qPCR), with triplicate cultures for each method (Fig. 1). To induce the osteogenic differentiation of the hBMSCs, the cultures were maintained in osteogenic media (OM), consisting of NO supplemented with 0.2 mM ascorbic acid (Amresco, USA), 10 mM β-Glycerol Phosphate (Sigma, USA) and 100 nM Dexamethasone (Sigma, USA) [7]. For each experiment, 7x104 cells were seeded in 12 multi-well plates (Falcon, USA) for an estimated 80% confluence. Each experiment comprised 12 cultures, 6 under osteogenic conditions and 6 controls under normal cultures conditions (NO). Medium was changed every 2-3 days.
2.3 Matrix mineralization
Alizarin Red S (ARS) stain was used to verify the state of osteogenic differentiation in terms of extracellular matrix mineralization [13]. Briefly, cells were fixed with 70% cold ethanol during 20 min, and then 500 µl of ARS (Sigma, USA) at 2% (W/V) pH 4.0 were added for 20 min. ARS solution was washed with ultra pure water and cultures were evaluated via phase contrast microscopy (Nikon Eclipse TS100, USA). The results were analyzed qualitatively based on the intensity of the staining and the extension of the ARS-stained positively areas (i.e. red spots).
2.4 RNA extraction and quantification
Total RNA was extracted using a spin-column based method and following the instructions of the manufacturer of the kit (RNeasy Mini Kit, Qiagen). Then, the extracted RNA was quantified using the Quant-iT RiboGreen Kit (Invitrogen, USA). The reactions were performed in a final volume of 100 µl by incubation in the real-time thermocylcer RotorGene 6000 (Corbett, Australia) for 2 min at 25 °C followed by fluorescence acquisition at 25 °C. A 5 point calibration curve (100 µg/ml to 500 µg/ml) was experiment, 7x104 cells were seeded in 12 multi-well plates (Falcon, USA) for an estimated 80% confluence. Each experiment comprised 12 cultures, 6 under osteogenic conditions and 6 controls under normal cultures conditions (NO). Medium was changed every 2-3 days. constructed using the standard RNA provided in the Kit, to transform fluorescence data into RNA concentration. All quantifications were performed in triplicate.
2.5 Quantitative RT-PCR
(RT-qPCR) A two step RT-PCR protocol was implemented for mRNA quantification. Briefly, 350ng of RNA were reverse transcribed using a QuantiTect Reverse Transcription Kit (Qiagen, USA) and following the instructions of the manufacturer. Reverse Transcription (RT) controls with no enzyme were prepared in order to detect potential contaminations with genomic DNA. cDNA and RT control samples were evaluated via quantitative PCR following the instructions of the manufacturer of the kit (QuantiTect SYBR Green, Qiagen). The reactions were performed with a final volume of 25 µl and using 300 nM for each primer (Table 1). The reactions were incubated in the real-time thermocycler RotorGene 6000 (Corbett, Australia). The PCR program consisted of an initial hold at 50°C for 2 min, followed by an activation step at 95°C for 15 min, and 45 cycles at 95°C for 15 sec, annealing at 60°C for 30 sec, and 72°C for 30 sec. Then, a melting curve was constructed by heating from 65°C to 95°C with temperature steps of 0.4ºC. All reactions included 0.25 U of uracil-DNA-glycosylase (Fermentas, USA) to avoid contamination with PCR products from previous reactions [14]. All quantifications were performed in duplicate. The analysis and estimation of the relative gene expression levels of the osteogenic markers was performed with the software qBase (i.e. the 2-ΔΔCT method) using cultures under non-osteogenic conditions at day 14 as reference (calibrators) and RPL13A as housekeeping gene [15]. Statistical analysis was performed using STATGRAPHICS Software (Statistical Graphics Corp., Version 5), with an unstacked One-way ANOVA at a 95% confidence level.
III. RESULTS
3.1 Cell differentiation and matrix mineralization
The hBMSCs cultures that were established from each of the three donors presented similar morphological and proliferation characteristics (proliferation data is not shown). After 2 days of the supplementation of the culture media the cells under osteogenic conditions exhibited morphological changes typical of the osteoblastic phenotype, and after 5 days the cells had acquired a polygonal osteoblast-like morphology (Fig. 2A) [5]. Cells in non-osteogenic conditions maintained an undifferentiated phenotype with a fibroblast-like morphology (Fig. 2B).
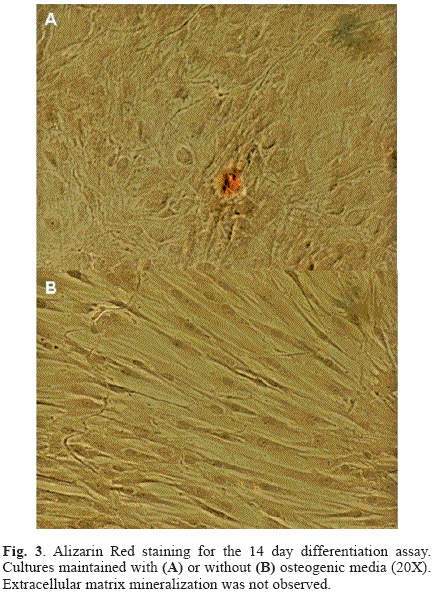
The results of the ARS staining of the 14 day differentiation assay are shown in Fig. 3. Matrix mineralization was not evident in the cultures under osteogenic conditions, with limited staining of small nodules in isolated regions (Fig. 3A), despite of the morphological changes indicative of the differentiation process that were observed in the first days of the osteogenic induction. However, the staining of the cultures of the 20 day differentiation experiment revealed an advanced process of calcium nodules formation characteristic of matrix mineralization (Fig. 4A-E). At both evaluation times, cultures under non-osteogenic conditions conserved the undifferentiated phenotype typical of these mesenchymal cells and did not present signs of matrix mineralization (Fig. 3B and Fig. 4F).
3.2 Expression of osteogenic markers
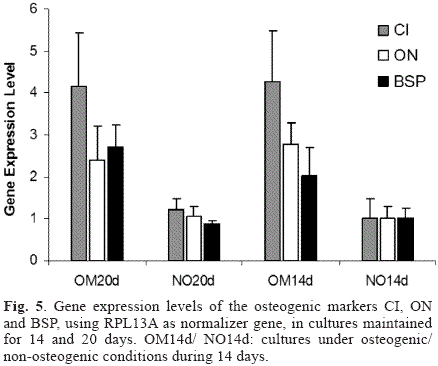
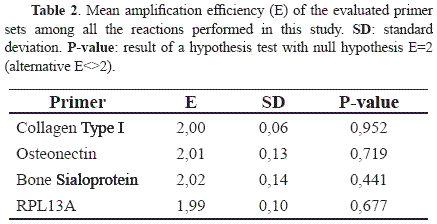
Gene expression levels of the osteogenic markers CI, ON and BSP under the different culture conditions evaluated in this study are shown in Fig. 5. The three osteogenic genes evaluated were up-regulated in the cultures under osteogenic conditions compared to the control cultures. However, when comparing the expression levels for each gene as a function of incubation time (i.e. OM20d vs. OM14d or NO20d vs. NO14d) no significant differences were found for CI (p=0.4314 for osteogenic cultures and p=0.103 for controls) and ON (p=0.1471 for osteogenic cultures and p=0.5465 for controls), but significance change was observed for BSP mRNA levels which were up-regulated in the cultures with osteogenic media (OM20d vs. OM14d, p=0.0017) and down-regulated in cultures under non-osteogenic conditions (p<0.00001). The stability of the expression levels of the normalizer gene RPL13A was validated under the osteogenic conditions used in this study (data not shown). Finally, since the mathematical model used for mRNA quantification relies on the assumption that the amplification efficiencies are 100% (i.e. E=2), the reaction efficiencies were calculated based on single-reaction data [16]. The statistical analysis demonstrated that the assumption was valid for all the genes evaluated, since all the p-values were above the significance level (p=0.05) as shown in Table 2.
IV. DISCUSSION
The osteogenic potential of the isolated mesenchymal cells was confirmed with the qualitative analysis based on the staining of the calcium accumulated in the extracellular matrix, which is one of the main events in the osteogenic differentiation process [17]. The evaluation time points, 14 and 20 days, are representative of different stages of this process as observed on the extent of the matrix staining, although at both times the major morphological changes characteristic of the osteoblastic phenotype had already occurred. The observation of the major mineralization events after 20 days of culture (Fig. 4) is in accordance with previous reports for mesenchymal stem cells suggesting similar osteogenic potentials [13, 18]. The results also demonstrated that the hBMSCs extracted from the femoral heads are capable of triggering the matrix mineralization process even after 5 passages in standard culture conditions, a characteristic observed in other hBMSCs models [19]. This allows to obtain higher cell yields through in vitro expansion with a concomitant increase in culture homogeneity [13], which favors flexibility and reproducibility in the experimental designs of posterior studies.
The mesenchymal model here presented is capable of conserving a stable undifferentiated phenotype (i.e. spindle-shape, fibroblast-like phenotype) when cultured under normal conditions for up to 10 passages (as so far evaluated, data not shown) and, after prolonged culture at high confluence, neither morphological changes nor signs of matrix mineralization are observed (Fig. 2, 3, 4). In contrast, upon osteogenic induction with culture media conditioned with traditional osteogenic supplements the cultures acquire a marked polygonal osteoblast-like phenotype and mineralize their extracellular matrix. The possibility to obtain a population of cells with osteogenic potential with a stable undifferentiated phenotype is important for studies of osteogenic induction (i.e. osteogenic differentiation as a result of the interaction with different biomaterials, application of physical forces or exposure to bioactive molecules) [11, 20-22]. For instance, embryonic stem cells models with known multipotentiality and hence osteogenic potential may have drawbacks in that scenario due to their tendency to mineralize their matrix even without induction, although at a minor extent than when cultured in osteogenic conditions [23-24].
Although the hBMSCs model used in this study has basal expression levels of the osteogenic markers (Fig. 5), only cultures under osteogenic conditions showed gene expression levels adequate for the induction of the osteogenic differentiation, as evidenced through the morphological changes and the mineralization of the extracellular matrix. In addition, the basal expression levels of these markers are important since they allow the use of relative quantification models of gene expression and therefore the precise quantification of the regulation of the osteogenic markers during the differentiation process as triggered by any treatment under evaluation [25].
The expression patterns of collagen type I, osteonectin and bone sialoprotein have been shown to be well-correlated to the osteogenic differentiation process and are involved in the early and intermediate stages of matrix formation and mineralization [26-27]. In this study, the expression levels of the three markers were up-regulated in the cultures with osteogenic media when compared to the controls (Fig. 5). Collagen type I (alpha 1) upregulation (maximum fold increase of 5.4) is a prerequisite for osteogenic differentiation and matrix synthesis, since this protein comprises over 90% of the organic material in the bone matrix [28]. Similarly, osteonectin and bone sialoprotein are up-regulated during the initial phase of matrix mineralization, with BSP considered as the main nucleator of hydroxyapatite crystal formation [20, 29]. This important role is in accordance with its higher regulation during the differentiation process, since only BSP presented statistically significant differences between cultures before and after mineralization (i.e. OM20d vs. OM14d). Moreover, correlation between the expression profiles of the ostegenic markers and the matrix mineralization process suggests that two fold or larger differences in the expression levels of these genes (Fig. 5) lead to phenotypic changes characteristic of the osteoblastic phenotype and to the onset of the mineralization process through bone nodule formation (Fig. 4).
A reduced number of studies have reported the extraction of BMSCs from the human femoral bone marrow. Most investigations with femoral heads have focused on trypsinization treatment of the trabecular bone or isolation of multipotent primary osteoblastic cells through explant cultures of trabecular bone [18, 30]. In 2005, Schutze and collaborators demonstrated the possibility of isolating multipotential bone marrow-derived mesenchymal stem cells from the processing of the trabecular bone in the femoral heads after repeated washings to release the cells from the bone plugs [31]. Pineda et al. 2007 reported the isolation of a cell population with mensenchymal characteristics using a variation of the mentioned protocol by means of mechanical disaggregation of the trabecular bone to release the cells from the femoral bone marrow [12]. In this study, this protocol was improved in terms of isolation efficiency through reduction of the surface culture area at the beginning of the primary culture (data not shown). In addition, the osteogenic potential of the cell population was verified demonstrating its capability of matrix mineralization upon osteogenic induction as well as the up-regulation in the gene expression levels of the evaluated osteogenic markers.
The isolated mesenchymal cells and the differentiation markers studied constitute an interesting model for the evaluation of the biological properties (e.g. osteoinductivity) of tissue engineering scaffolds and different biomaterials or bioactive molecules. The demonstrated stable undifferentiated phenotype in non-treated cultures is an interesting characteristic of the presented mensenchymal model, since it would allow the measurement of the osteoinductivity of different materials with independence of the phenotypic changes associated with culture time. Although mineralization studies with staining methods are simple and very informative, they can not be used often when the differentiation process takes place in a complex microenvironment (usually opaque), reducing the applicability of conventional optical imaging techniques or introducing non-specific staining in the system. For these reasons, the possibility of studying the differentiation process through the quantification of the expression levels of specific osteogenic markers turns out to be of high importance. Finally, the hBMSCs model presented in this study has limited applications on regenerative medicine or tissue repair due to the nature of the biological samples required for the isolation protocol.
V. CONCLUSION
The human bone marrow mensenchymal stem cells obtained in this study presented a stable undifferentiated phenotype under normal culture conditions after prolonged cell culture, and maintained their osteogenic potential after several passages as demonstrated by their capability to mineralize the extracellular matrix and the respective upregulation of the bone markers collagen type I, osteonectin and bone sialoprotein upon osteogenic induction after 5 passages. This hBMSCs extraction model presents several advantages compared to the bone marrow aspirates: 1) limited ethical issues for obtaining biological samples, 2) greater availability of tissue from total hip replacement surgeries, and 3) although not explored in this study, the possibility of also establishing osteoblast primary cultures from trabecular bone explants. The overall characteristics of the isolated stem cells, mainly their long-term osteogenic potential, stable undifferentiated phenotype, and basal expression levels of the studied osteogenic markers are desirable for in vitro evaluation of the osteoinductivity of different biomaterials, bioactive molecules or tissue engineering scaffolds.
REFERENCES
[1]. Hung S., Chen N., Hsieh S., Li H., Ma H., Lo W. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells, 20, 249-258, 2002. [ Links ]
[2]. Sekiya I., Larson B., Smith L., Pochampally R., Cui J., Prockop D.J. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells, 20, 530-541, 2002. [ Links ]
[3]. Grayson W.L., Ma T., Bunnell B. Human mesenchymal stem cells tissue development in 3D PET matrices. Biotechnology Progress, 20, 905-912, 2004. [ Links ]
[4]. Kim H., Kim U., Vunjak-Novakovic G., Min B., Kaplan D.L. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials, 26:4442-4452, 2005. [ Links ]
[5]. Meinel L., Karageorgiou V., Hofmann S., Fajardo R., Snyder B., Li C., Zichner L., Langer R., Vunjak-Novakovic G., Kaplan D.L. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. Journal of Biomedical Materials Research Part A, 71A, 25-34, 2004. [ Links ]
[6]. Meinel L, Karageorgiou V, Fajardo R., Snyder B., Shinde-Patil V., Zichner L., Kaplan D., Langer R., Vunjak-Novakovic G. Bone Tissue Engineering Using Human Mesenchymal Stem Cells: Effects of Scaffold Material and Medium Flow. Annals of Biomedical Engineering, 32(1):112-122, 2004. [ Links ]
[7]. Meinel L., Hofmann S., Betz O., Fajardo R., Merkle H.P., Langer R., Evans C.H., Vunjak-Novakovic G., Kaplan D.L. Osteogenesis by human mesenchymal stem cells cultured on silk biomaterials: Comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials, 27, 4993-5002, 2006. [ Links ]
[8]. Caterson E., Nesti L., Danielson K., Tuan R. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Molecular Biotechnology, 20, 245-256, 2002. [ Links ]
[9]. De Oliveira P.T., Nanci A. Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials, 25, 403-413, 2004. [ Links ]
[10]. Sumanasinghe R.D., Bernacki S.H., Loboa E.G. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue Engineering, 12, 3459-3465, 2006. [ Links ]
[11]. Catelas I., Sese N., Wu B., Dun J., Helgerson S. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Engineering, 12(8), 2385-2396, 2006. [ Links ]
[12]. Pineda C., García F., Gallego D., Higuita N., López L.E., Sarassa C., Agudelo P., Hansford D.J., Jaramillo L. Isolation of human bone marrow mesenchymal stem cells and their potential applications for biomaterials evaluation. Proceedings of the III symposium about Biofactories, Medellín, Colombia, August 2007. [ Links ]
[13]. Colter D.C., Sekiya I., Prockop D.J. Identification of a subpopulation of rapidly selfrenewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proceedings of the National Academy of Sciences of the United States of America, 98(4), 7841-7845, 2001. [ Links ]
[14]. Kleiboeker S.B. Quantitative assessment of the effect of uracil-DNA glycosylase on amplicon DNA degradation and RNA amplification in reverse transcription-PCR. Virology Journal, 2:29, 2005. [ Links ]
[15]. Hellemans J., Mortier G., De Paepe A., Speleman F., Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biology, 8(2):R19, 2007. [ Links ]
[16]. Ramakers C., Ruijter J.M., Lekanne Deprez R.H., Moorman A.F.M. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience Letters, 339, 62-66, 2003. [ Links ]
[17]. Friedman M.S., Long M.W., Hakenson K.D. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. Journal of Cellular Biochemistry, 98, 538-554, 2006. [ Links ]
[18]. Noth U., Osyczka A.M., Tuli R., Hickok N.J., Danielson K.G., Tuan R.S. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. Journal of Orthopaedic Research, 20, 1060-1069, 2002. [ Links ]
[19]. Kulterer B., Fried G., Jandrositz A., Sanchez-Gabo F., Prokesch A., Paar C., Scheideler M., Windhager R., Preisegger K., Trajanoski Z. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genomics, 8:70, 2007. [ Links ]
[20]. Frank O., Heim M., Jakob M., Barbero A., Schafer D., Bendik I., Dick W., Heberer M., Martin I. Real-time quantitative RT-PCR analysis of human bone marrow stromal cells during osteogenic differentiation in vitro. Journal of Cellular Biochemistry, 85, 737-746, 2002. [ Links ]
[21]. Tsukahara S., Ikeda R., Goto S., Yoshida K., Mitsumori R., Sakamoto Y., Tajima A. Tumour necrosis factor α-stimulated gene-6 inhibits osteoblastic differentiation of human mesenchymal stem cells induced by osteogenic differentiation medium and BMP-2. The Biochemical Journal, 398, 595-603, 2006. [ Links ]
[22]. Cho H., Park H.T., Kim Y.J., Bae Y.C., Suh K.T., Jung J.S. Induction of osteogenic differentiation of human mesenchymal stem cells by histone deacetylase inhibitors. Journal of Cellular Biochemistry, 96, 533-542, 2005. [ Links ]
[23]. García F., Zapata N.M., López L.E., Londoño C. Characterization of a multipotential bovine cell source and its application for biomaterials evaluation. Proceedings of the IV Latin American Congress on Biomedical Engineering 2007, Bioengineering Solutions for Latin America Health 2007, 18: 1211-1215. [ Links ]
[24]. Bielby R.C., Boccaccini A.R., Polak J.M., Buttery L.D.K. In Vitro differentiation and in Vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Engineering, 10(9), 1518-1525, 2004. [ Links ]
[25]. Livak K.J, Schmittgen T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods, 25(4): 402-408, 2001. [ Links ]
[26]. Silva W.A., Covas D., Panepucci R.A., Proto-Siqueira R., Siufi J., Zanette D., Santos A., Zago M.A. The Profile of Gene Expression of Human Marrow Mesenchymal stem cells. Stem Cells, 21: 661-669, 2003. [ Links ]
[27]. Mygind T., Stiehler M., Baatrup A., Li H., Zou X., Flyvbjerg A., Kassem M., Bunger C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials; 28, 1036-1047, 2007. [ Links ]
[28]. Bosetti M., Zanardi L., Hench L., Cannas M. Type I collagen production by osteoblast-like cells cultured in contact with different bioactive glasses. Journal of Biomedical Materials Research, 64A, 189-195, 2003. [ Links ]
[29]. Kreke M.R., Huckle W.R., Goldstein A.S. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone, 36, 1047-1055, 2005. [ Links ]
[30]. Sakaguchi Y., Sekiya I., Yagishita K., Ichinose S., Shinomiva K., Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood, 104, 2728-2735, 2004. [ Links ]
[31]. Schutze N., Noth U., Schneidereit J., Hendrich C., Jakob F. Differential expression of CCN-family members in primary human bone marrow-derived mesenchymal stem cells during osteogenic, chondrogenic and adipogenic differentiation. Cell Communication and Signaling, 3:5, 2005. [ Links ]