Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Ingeniería Biomédica
Print version ISSN 1909-9762
Rev. ing. biomed. vol.3 no.5 Medellín Jan./June 2009
DEVELOPMENT OF AN ACTUATED CABLE ORTHOTIC GLOVE TO PROVIDE ASSISTANCE OF FINGER EXTENSION TO STROKE SURVIVORS
DESARROLLO DE UN GUANTE ORTÓTICO PARA PROVEER ASISTENCIA EN LA EXTENSIÓN DE LOS DEDOS A PACIENTES QUE HAN SUFRIDO DERRAME CEREBRAL
José Mauricio Ochoa1, Derek Kamper2
1 Biomedical Engineering Program. Escuela de Ingeniería de Antioquia-Universidad CES, Colombia.
2 Sensory Motor Performance Program. Rehabilitation Institute of Chicago, Department of Biomedical Engineering, Illinois Institute of Technology, EE.UU. Contact e-mail: bmjomau@eia.edu.co.
Received July 14, 2008. Accepted April 28, 2009.
ABSTRACT
An externally actuated glove, controlled by a microprocessor, is being developed to assist finger extension in stroke survivors. The goal of this device is to allow repeated practice of specific tasks for hand therapy in the home environment. The device allows the user three control modes: voice recognition, electromyography or manual. These modes can be used either independently or combined according to the needs of the user. Both position and force feedback are available for control and safety. Initial testing of the prototype has shown promising performance.
KEY WORDS: Electromyography, feedback, Hand orthosis, Hemiparesis, Rehabilitation engineering, Voice recognition.
RESUMEN
Se presenta el desarrollo de un guante activado externamente y controlado por un microprocesador para asistir la extensión de los dedos en pacientes con derrame cerebral. La meta del dispositivo es permitir la repetición de tareas específicas para realizar terapia de la mano en un ambiente casero. El usuario puede controlar el dispositivo por tres medios diferentes: reconocimiento de voz, electromiografía o manualmente. Estos medios pueden ser usados tanto independientemente como en combinación según las necesidades del paciente. Para el control y la seguridad, se tiene retroalimentación de posición y de fuerza. Las pruebas iniciales del prototipo han demostrado un desempeño prometedor.
PALABRAS CLAVE: Electromiografía, Ortosis de mano, Hemiparesis, Ingeniería de rehabilitación, Reconocimiento de voz.
I. INTRODUCTION
Most of the patients diagnosed with stroke suffer from impaired arm function, and approximately one-third of all patients experience chronic hemiparesis [1-2]. Chronic hemiparesis is especially prevalent in the distal upper extremity. Finger extension is the most impaired function [3]; a spontaneous finger flexion appears a few weeks after the cerebrovascular accident [4]. Two factors contribute to the spontaneous finger flexion. First, the long flexors of the fingers, flexor digitorum superficialis (FDS) and flexor digitorum profundus (FDP) are often hyperexcitable [5]. Second, the finger extensors tend to be weakened, especially in severely impaired individuals [6-7]. Both factors lead to the stereotypical flexed finger posture [4].
Nevertheless, finger extension is sometimes preserved in stroke survivors, although weakened [8]. In a recent study [4,7] it was demonstrated that less than 30% of the severely impaired stroke subjects could perform active isometric extension. In absolute terms, metacarpalphalangeal (MCP) extension torque was only 7% of the MCP flexion torque.
This fact confirms the need of an assistive device for finger extension, adaptable to the patient's individual abilities to activate his own finger extensors. There are several commercial devices available which could provide assistance for finger extension. However, these devices generally provide only one control mechanism. The Saeboflex (Saebo, Inc., Charlotte, NC) is a custom dynamic hand orthosis featuring a spring loaded finger extension system; the spring system assists in re-opening the digits following functional grasping, but motion of the distal interphalangeal (DIP) joints is occluded and movement of the MCP joints is limited [4,9]. The Hand Mentor (Kinetic Muscles Inc., Tempe, AZ) creates combined flexion/extension of the wrist and fingers, but restricts movement of the proximal interphalangeal (PIP) and DIP joints [4]. Cybergrasp (Immersion Corporation, San Jose, CA) provides independent extension forces to each digit, but it can be considered large and restricts arm movement [4].
An cable-actuated orthotic glove controlled by a microprocessor is proposed, in which the glove is connected to a motor shaft through a cable. The microprocessor is able to detect the electromyographic (EMG) signals from the extensor muscles and use them to activate the motor to open the hand. If the power of the EMG signal is not strong enough, a voice-recognition algorithm was implemented as a secondary mechanism for the same purpose. In addition, a manual control mechanism provides a third control option in order to make the assistive device more reliable. This device is relatively simple, and targeted toward severely impaired patients who need to work on spherical grasp and release. The proposed device utilizes the asymmetry between the MCP extension and the MCP flexion to reduce complexity and consequently reduces cost by providing assistance for finger extension, and relying on residual finger flexion for performing grasping tasks.
II. MATERIALS AND METHODS
2.1 Hardware
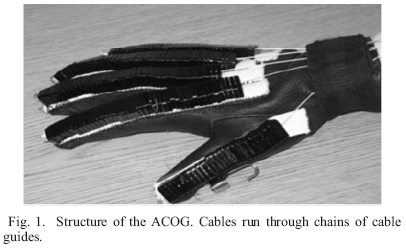
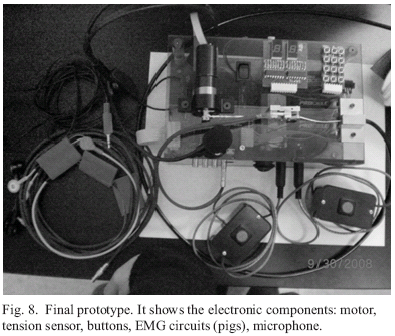
The actuated cable orthotic glove (ACOG) provides assistance of finger extension in order to dorepeated specific tasks. The ACOG is portable and actuated by a single DC motor worn in a small beltpack.
Extension torques are produced by tendon-like cables running across the dorsal side of the glove through chains of custom-fabricated Delrin® pieces which serve as cable guides (Fig. 1). These guides not only maintain the line of force and moment arm of the cable but also prevent hyperextension of joints, which can be a major issue in stroke survivors, especially when applying external forces. The cables from the 5 digits are combined into a single cable (cable displacements necessary to produce full extension of each digit are sufficiently similar across digits to permit use of a single actuator) which then travels through a cable guide to the shaft of the actuator, a DC Micromotor (DC-Micromotors, Gearhead 20/1, Encoders IE2-512 ;1724, Faulhaber, Inc.).
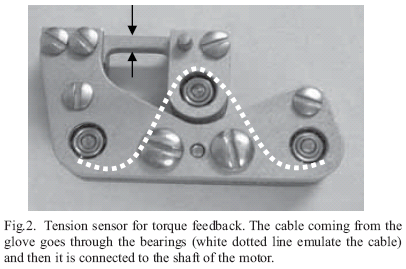
With a gear head, the selected motor is able to produce the desired force with a maximum of 130 N, needed to open the hand. A motor encoder supplies information about the number of degrees between the thumb and the other fingers, which is proportional to the cable lengths. Feedback of torque applied to the cable is provided with an in-line tension sensor, useful to know how much force is pulling the hand. The sensor has two strain gages (Model No.SGD-1.5/120-LY11) placed on a small aluminum piece, one at the top and the other at the bottom, for measuring the deformation (black arrows, Fig. 2). The output of these two strain gages is conditioned and then read as the tension in the cable (designed in the Hand Rehabilitation Laboratory at the Rehabilitation Institute of Chicago, Fig. 2).
2.2 Software
The motor is controlled by a small microprocessor board, programmed with the Dynamic C software (Version 10.11, Rabbit Semiconductor, Inc) with analog-to-digital (A/D) conversion, pulse-width modulation (PWM) capabilities and quadrature decoder channels (QDC) (RabbitCore RCM4500W, RABBIT Semiconductor Inc, Davis, CA). A Tmote sky (Ultra low power IEEE 802.15.4 compliant wireless sensor moduleMoteiv Corp., US) was used in combination with an in-line load cell in the selfactuated version of the orthosis [10].
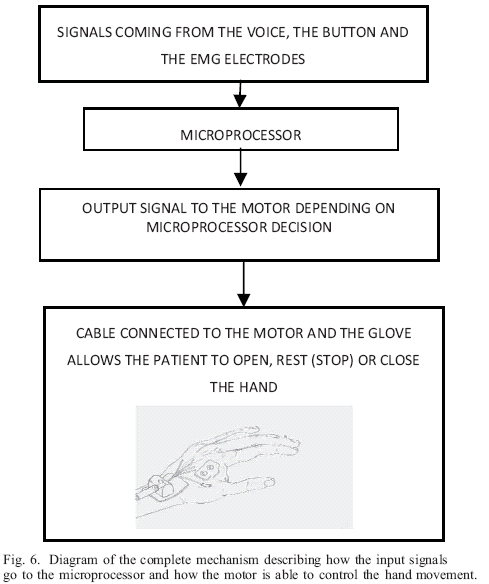
Fig.3 shows a flow chart of the algorithm. It describes how the action of saying a word ("open", "close" or "stop"), activating a muscle, or pushing a button (manual mode) can control the system. With each word pronounced (the word only has to be pronounced once) the voice recognition system provides as an output a number that is read by the microprocessor and hence, the motor driven glove runs one way or the other depending on the word command; with the EMG signals the RabbitCore compares which signal (EMG signals from extensor and flexors) has the biggest amplitude in order to determine the command to be executed. Finally, if the manual control button is pressed, the motor cycles through three possible states: extension (hand opening), flexion (hand closing), and the stop state. During flexion, the tension sensor reads the force applied, gives the signal to the motor to release the cable and let the patient close the hand voluntarily. The motor speed controlled by the pulse width modulation (PWM) frequency, is set proportional to the sensor reading and to the error between the desired angle (the limit of either flexion or extension, these limits are defined at the beginning of the training session through a second button) and the actual angle read by the encoder.
The ACOG controlled by the microprocessor is able to use either force or position servocontrol depending on the needs of the user. Position feedback, from the encoder data is used to generate a desired amount of digit extension. Force feedback, utilizing the tension sensor data, provides a desired level of assistance (e.g., motor becomes active when cable force exceeds a certain level). Appropriate feedback will be defined for each stroke patient after the research therapist evaluation. The flexibility of the system described above allows the user to control the degree of assistance. This degree can certainly vary from subject to subject. For example, one type of input is the EMG signal of a finger extensor muscle, extensor digitorum communis (EDC).
2.3 Circuit
Three small printed circuit boards were made to amplify the EMG signal from passive electrodes and calculate filtered, rectified EMG signal as a measure of the EMG amplitude. The outputs of these PC boards are read by the RabbitCore through A/D conversion channels. The amplitude is compared with the signal from another muscle, such as the FDS muscle, to determine the level of assistance during extension (Fig. 4).
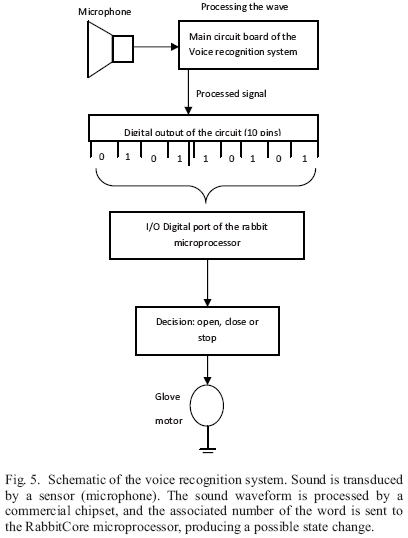
The voice recognition control algorithm was implemented using a commercially available chipset. The chipset manufactured by Electronic ExpressTM [11] was selected to be incorporated into the ACOG. The output of the voice recognition circuit consists of two 4-bit binarycoded decimal (BCD) numbers. This BCD information is shown on two digital displays. Whenever a word is detected, the circuit uses the digital display to output the word number it has recognized. If the word is not recognized by the system, it outputs an unrecognized/ error code. The designed interface incorporates a MICROCHIP microcontroller. The pre-programmed microcontroller's first job is to determine if a word has been said. To do so, a comparator amplifier is used. A reference voltage for the comparator is generated using a voltage divider made up of resistors.
Once a word has been detected, it is necessary for the interface to read the BCD output from the voice recognition circuit. By using the high and low digit BCD nibbles, it is possible to distinguish trained target words [12]. The interface has 10 outputs (the information that goes into the two digital displays) which are connected to ten I/O channels in the RabbitCore microprocessor (Fig. 5). Identification of a given command is accomplished by reading these values.
III. RESULTS
The Dynamic C software which implements the control paradigms in the microprocessor (EMG pattern recognition, voice recognition and manual control) and the glove were tested successfully by means of voice commands of 2 healthy subjects. The proposed device has not yet been tested with stroke patients. During the recording trial subjects attempted to open and close their hands as many times as possible within a period of 10 seconds.
Future evaluation of the performance of the EMG pattern recognition program code will use: EMG signals recorded over flexor digitorumsuperficialis (FDS) and EDC of 3 stroke patients in stage 3 on the Chedoke McMaster Stroke Assessment scale [13], aiming to determine the difference in signal amplitude, detected by the microprocessor of an active and an inactive muscle.
The software and glove final performance met all requirements, as expected, when being controlled by voice and EMG signals of healthy individuals. The aperture and closure of the subjects' hands were properly achieved and followed the programmed code. The buttons worked as a security mode in case of any of the other input mechanisms failed.
Fig.7 shows the EMG signals of the stroke participants after rectification and filtering in MATLABTM to simulate the actual output from the printed circuit boards for ACOG. EMG signals from the EDC and FDS muscles were recorded for two of the patients (Fig 7.a. and b.). FDS is largely active during the attempt of flexion and EDC is largely active during the attempt of extension independently of the voltage magnitude displayed for both of these trials (the voltage range between an inactive muscle and an active muscle can vary from patient to patient). In Fig. 7(a. and b.) it is possible to differentiate a range in which the muscle is active and one where it is inactive. On the other hand, in the last participant (Fig 7 c.) it is difficult to discern, based on the EMG signals, when flexion and extension were intended to occur. Consequently, it is doubtful that EMG alone could be used by this subject to control the ACOG; voice recognition, however, could be used in conjunction with the EMG signal.
IV. DISCUSSION
Several hand exoskeletons have been developed in various research laboratories, but technical issues seem to have either limited their development or precluded their application to rehabilitation [4,14-17]. For example, the University of California-Irvine uses pneumatic cylinders to control wrist and MCP flexion/extension, but does not actuate the PIP and DIP joints [4,18]. The Rutgers Master II-ND (RMII) is a device which has been used in clinical trials with stroke survivors [4,19]. The RMII uses custom made pneumatic cylinders on the palmar side of the hand to push the fingertips into extension. The RMII, however, was intended for use in a virtual environment so it is not possible to grasp real objects while wearing the device. Additionally, finger flexion is limited by the length of the cylinders residing within the palmar space; PIP flexion angle is limited to 45° [4,12].
The device proposed in the present study, with three developed input mechanisms, showed a satisfactory performance. Moreover, voice recognition was reliable since the system can quickly be trained for each patient (30 s). The EMG pattern recognition algorithm may be challenged by signals from patients with severe tone due to the co-activation of flexor and extensor muscles.
An extra button had to be implemented for recording the range of motion for each of the patients because some of them felt pain when the hand was being open more than a fixed angle. This extra button was included in the software through a second interruption. In this case, the range of motion had to be defined in the first cycle of the algorithm.
To implement the EMG as a control signal for users with severe spasticity where EMG might be very weak, the microprocessor can be programmed to have the voice recognition pathway as a security mode. Thus the device can be controlled with the muscle signals from the impaired arm locked to the desired movement. As an example: if the user says the word open the microprocessor will only read the signal from the extensors muscles and viceversa. Doing so the system will not open or close the hand until the patient activate the right muscle.
The developed device is entirely portable so the user can take the glove home and perform regular daily activities without requiring going to a rehabilitation hospital for therapy. Also, the posibility of controlling the glove with both voice and movement of muscles will force the patients to think about the intended function (openhand, closehand); or to activate the impaired muscles if they are driving it with EMG signals adding an extra exercise to the rehabilitation therapy.
V. CONCLUSION
Initial algorithm and electronic designs have been implemented and show promise and a potential performance of the proposed device when used with stroke survivors.
The next step in the development of the ACOG, controlled by microprocessor, is to build and design a portable equipment to hold together all the circuits and other components of the final design, in order to ensure portability and ease of use of the device.
After having a final design, a rigorous test and characterization of the developed prototype is required, in terms of its feasibility and performance. Additionally, patients should exhibit a good control of the motorized orthosis.
In future work, given the expected ease of use and portability of the device, therapeutic trials will be conducted at home in accordance with the regimen established by a research occupational therapist.
ACKNOWLEDGMENT
The authors would like to thank the SMPP staff that helped during the time this project was carried out at the Rehabilitation Institute of Chicago. This work was supported by grants from the Coleman Foundation and the National Institute on Disability and Rehabilitation Research (H133E070013-Rehabilitation Engineering Research Center).
REFERENCES
[1] Parker V.M., Wade D.T., Langton R. Loss of arm function after stroke: measurement, frequency, and recovery. Int Rehabil Med, 8(2), 69-73, 1986. [ Links ]
[2] Gray C.S., French J.M., Bates D., Cartlidge N.E., James O.F., Venables G. Motor recovery following acute stroke. Age Ageing, 19(3), 179-184, 1990. [ Links ]
[3] Trombly C.A. "Stroke" in occupational therapy for physical dysfunction, ed. C.A. Trombly, Baltimore: Williams and Wilkins, 1989, 454-471. [ Links ]
[4] Kamper D., Tsoupikova D., Vick R., Stoykov N., Connelly L., Jia Y. Bringing hand rehabilitation into new environments. Grant proposal, 2007. [ Links ]
[5] Kamper, D.G., Rymer W.Z. Quantitative features of the stretch response of extrinsic finger muscles in hemiparetic stroke. Muscle and Nerve 23(6), 954-961, 2000. [ Links ]
[6] Cruz, E.G., Waldinger, H.C., Kamper, D.G. Kinetic and kinematic workspaces of the index finger following stroke. Brain, 128(Pt 5), 1112-11121, 2005. [ Links ]
[7] Kamper D.G., Fischer H.C., Cruz E.G., Rymer W.Z. Weakness is the primary contributor to finger impairment in chronic stroke. Arch Phys Med Rehabil, 87(9), 1262-9, 2006. [ Links ]
[8] Kamper D.G., Rymer W.Z. Impairment of voluntary control of finger motion following stroke: role of inappropriate muscle coactivation. Muscle and nerve, 24(5), 673-681, 2001. [ Links ]
[9] Farrell J., Hoffman H., Snyder J., Giuliani C., Bohannon R. Orthotic aided training of the paretic upper limb in chronic stroke: Results of phase 1 trial. NeuroRehabilitation, 22(2), 99-103, 2007. [ Links ]
[10] Villa J., Petroff N. Development of an electro-mechanically controlled hand orthosis for assisting finger extension in stroke survivors. Revista Ingeniería Biomédica, 2, 48-54. 2007. [ Links ]
[11] Images Scientific Instruments: build a speech recognition circuit. Last consulted 7 March, 2008 at: http://www.imagesco.com/articles/hm2007/SpeechRecognitionTutorial01.html. [ Links ]
[12] Bouzit M., Burdea G., Popescu G., Boian R. The Rutgers Master II-new design force-feedback glove. IEEE/ASME Transactions on mechatronics, 7(2), 256-263, 2002. [ Links ]
[13] Gowland C., Van Hullenaar S., Torresin W., Moreland J., Vanspall B., Barrecca S. Chedoke McMaster stroke assessment: development, validation and administration manual. Hamilton, Canada: Chedoke-McMaster Hospitals and McMaster University, 1995. [ Links ]
[14] Shields B.L., Main J.A., Peterson S.W., Strauss A.M. An anthropomorphic hand exoskeleton to prevent astronaut hand fatigue during extravehicular activities. IEEE Trans Syst Man Cybern A Syst Hum, 27(5), 668-73, 1997. [ Links ]
[15] Nakagawara S., Kajimoto H., Kawakami N., Tachi S, The Touch thimble: providing fingertip contact feedback during point-force haptic interaction. IEEE International Conference on Robotics and Automation, 2667-2672, April 2005. [ Links ]
[16] DiCicco M., Lucas L., Matsuoka Y. Comparison of control strategies for an EMG controlled orthotic exoskeleton for the hand, IEEE International Conference on Robotics and Automation 1622-1627, 2004. [ Links ]
[17] Jugenheimer K.A., Hogan N., Krebs H.I. A Robot for hand rehabilitation: a continuation of the MIT-MANUS Neuro-Rehabilitation Workstation, ASME International Design Engineering Technical Conference IDETC/CIE, Pittsburgh, September, 2001. [ Links ]
[18] Takahashi C.D., Der-Yeghiaian V.H., Cramer S.C. A robotic device for hand motor therapy after stroke. Rehabilitation Robotics, IEEE 9th International Conference on Rehabilitation Robotics, 17-20, 2005. [ Links ]
[19] Jack D., Boian R., Merians A.S., Tremaine M., Burdea G.C., Adamovich S.V., Recce M., Poizner H. Virtual reality-enhanced stroke rehabilitation. IEEE Trans Neural Syst Rehabil Eng, 9(3), 308-318, 2001. [ Links ]