Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Ingeniería Biomédica
Print version ISSN 1909-9762
Rev. ing. biomed. vol.8 no.16 Medellín July/Dec. 2014
TOPOGRAPHICAL AND STRUCTURAL EVALUATION OF DIATHERMY PENCILS EMPLOYED ON AESTHETIC SURGERY SUBJECTED TO FOUR REPROCESSING CYCLES USING THE EIA/CES "QUALY" APPROACH
EVALUACIÓN TOPOGRÁFICA Y ESTRUCTURAL DE LÁPICES DE DIATERMIA EMPLEADAS EN CIRUGÍA ESTÉTICA, SOMETIDOS A CUATRO CICLOS DE REPROCESADO UTILIZANDO EL ENFOQUE DE EIA/CES "QUALY"
AVALIAÇÃO TOPOGRÁFICA E ESTRUTURAL DE LÁPIS DE DIATERMIA USADOS EM CIRURGIA COSMÉTICA SOFRIDO QUATRO CICLOS DE REPROCESSAMENTO USANDO A ABORDAGEM DE EIA/CES "QUALY"
Sebastián Torres1, Yesid Montoya2, Claudia E. Echeverri2, Pablo Giraldo3, Lina Tapia4
1 Administrative Department of Science Technology and Innovation (Colciencias), Young Researcher program, Bogotá, Colombia.
2 Biomaterials Laboratory, Department of Biomedical Engineering, Engineering School of Antioquia -EIA-, CES University, Sabaneta, Colombia. Dirección para correspondencia: pfyemon@eia.edu.co (YM).
3 Biomedical Engineering, Maintenance Department, IQ Interquirofanos, Medellín, Colombia.
4 Medical Instrumentation, Central Sterile Supply Department, IQ Interquirofanos, Medellín, Colombia.
Recibido 4 de septiembre de 2014. Aprobado 29 de octubre de 2014.
ABSTRACT
Diathermy pencils are one of the most employed medical devices around the world, this condition increases its probability to be reprocessing in patients; diathermy pencil has different parts (cord, tip, handgrip, current cables), which are composed of different kind of materials. In order to identify the medical device behavior is necessary to characterize all of those materials in the instrument. An exploration to determinate topographic surface defects and structural movements in each material was performed, with the intention of evaluated the reuse cycles effects on the medical device. The experiment was performing using the EIA/CES "QUALY" approach [1]. The study concludes that four reprocessing cycles induce some changes that are not statistically representative. Surface defects were detected in the cord; also an aggressive superficial waste after four reprocessing cycles in the tip was observed. We do not observe structural changes in the polymeric chain of the cord.
KEYWORDS: Reprocessing Medical Devices (RUDM); Diathermy Pencils; Optical Microscopy; Spectroscopy; Wear.
RESUMEN
Los lápices diatermia son uno de los dispositivos médicos más empleados en todo el mundo, condición que aumenta su probabilidad de ser reprocesados con pacientes. El lápiz diatermia tiene diferentes partes (cable, punta, empuñadura, cables de corriente), que están compuestas de diferentes tipos de materiales. Con el fin de identificar el comportamiento de dispositivos médicos es necesario caracterizar todos esos materiales en el instrumento. Se efectuó una exploración de los defectos en la superficie topográfica y determinados movimientos estructurales en cada material, con el propósito de evaluar los efectos de los ciclos de reutilización sobre el dispositivo médico. El experimento se realizó mediante el enfoque "QUALY" EIA/CES [1]. El estudio concluye que cuatro ciclos de reprocesado implican algunos cambios que no son estadísticamente representativos. Se detectaron defectos en la superficie del cable; igualmente, se observó un residuo superficial agresivo después de cuatro ciclos de reprocesamiento en la punta. No se observaron cambios estructurales en la cadena polimérica de la cuerda.
PALABRAS CLAVE: Reprocesamiento de dispositivos médicos (RUDM); Lápices diatermia; Microscopía óptica; Espectroscopia; Uso.
SUMÁRIO
Os lápis de diatermia são um dos dispositivos mais utilizados em todo o mundo médico, uma condição que aumenta a sua chance de ser reprocessados com os pacientes. O lápis de diatermia tem diferentes partes (cabo, ponta, aderência, linhas de energia), que são compostas de materiais diferentes. A fim de identificar o comportamento de dispositivos médicos é necessário caracterizar todos tais materiais no instrumento. Uma varredura de defeitos foi realizado na superfície topográfica e certos movimentos estruturais em cada material, com o objetivo de avaliar os efeitos dos ciclos de reutilização no dispositivo médico. O experimento foi realizado utilizando o EIA/CES [1] abordagem "QUALY". O estudo conclui que quatro ciclos de reprocessamento envolvem algumas mudanças que não são estatisticamente representativa. Os defeitos foram detectados na superfície do cabo; do mesmo modo, um resíduo de superfície agressivo foi observada após quatro ciclos na ponta reprocessamento. Não foram observadas alterações estruturais na cadeia do polímero da corda.
PALAVRAS-CHAVE: Reprocessamento de dispositivos médicos (RUDM); Lápis de diatermia; A microscopia óptica; espectroscopia; Uso.
I. INTRODUCTION
The Reprocessing of Medical Devices (RDM) is an implementation of a device after an initial use, cleaning procedures (physical or chemical), sterilization process, packaging devices and storage inventory. This practice is common in providing health services institutions around the world and continues to be a risky practice for the patient if there not a technical and scientific support to verify it. Previous work suggests that the RDM is an alternative for large demands specialized procedures in patients, especially in areas where the medical devices distribution is not efficient, other reason is the constantly changing due to frequent updates of medical technologies. Some works on this topic are focused exclusively on the scientific description without attaching clinical implications or effects arising from the RDM procedure. It is reported that one of the most reused devices is the electrosurgical active electrode (Diathermy pencil) [2, 3, 4, 5, 6]; the main reason for reprocessing this device, is the high volume of surgeries relative to the institutions cost/benefits relation. This research involved the characterization of the constituent material of a medical device; the study was performed on the cord, cutting blade (tip) and current cables. The cord is constituted by a medical polymer that is describe as a hard - brittle material, but with the addition of plasticizers, it can be made flexible and soft; however, can arise problems for long-term applications because the human body can extract the plasticizers. Although these plasticizers have low toxicities [7], the properties of the polymers can increase the stiffness of the rubber mixture [8]. The tip is generally made by several types of stainless steels; in practice the most common is 316L (ASTM F138, F139), grade 2. This steel has less than 0.030% (wt.%) carbon in order to reduce the possibility of in vivo corrosion. The "L" in the designation 316L denotes low carbon content. The 316L alloy is predominantly iron (60-65%) with significant alloying additions of chromium (17-20%) and nickel (12-14%), plus minor amounts of nitrogen, manganese, molybdenum, phosphorus, silicon and sulfur [9]. Copper alloys mostly constitute current cable; they have high resistance against corrosion in compare with steel; the best-known traditional types are brass, where tin is a significant addition. Copper alloy tends to be substituted, especially by museums Copper; this is a copper trace conductive material that thick will have a temperature increase of nearly 1°C when it carries 800 µA. If several traces carry similar currents, possibly causing thermal damage to the tissue [10].
Some previous works suggest forms to make the evaluation [11, 12]. Our experiment was conducted for twenty-one pencils in three standards tests; probabilistic methods were used to observe the variance in spectral properties; logistic regression were used to determinate topographical changes. All of the data were analyzing under the same experimental design. General objective was determined significant differences for four reprocessing cycles.
II. MATERIALS AND METHODS
2.1. Method for characterization
The results were obtained performing the EIA/CES "QUALY" approach.
2.2 Diathermy Pencils
Twenty-one diathermy pencils for electro surgery procedures from Valley Lab (Boulder, CO, USA) [13].
2.3 Use and sterilization process
Twenty-one pencils were separate in five groups coded as, R0, R1, R2, R3 and R4. Four pencils were assigned to each group and were used the number of times of its code (example: R4, group of pencils with four reprocessing cycles). The first group (R0) has one pencil, with no uses on patient, and was considered as a control specimen, the others groups were employed in patients, washed and sterilized through Ethylene Oxide (EO) under 38 °C, 0.089MPa (13 psi), 0.6L (EO) and 120 minutes with 60 minutes of aeration per diathermy pencil; at last the devices were packages, labeling and sending to the biomaterials laboratory. In order to identify the device sample as analysis specimens, each device was packed and identified as "E" for the pencil and "R" for reuse (example: E1R1, pencil "one" reuse "one").
2.4 Characterization tests
Each sample was taken from its package, the tip was separate in an individual package for a stereoscopy test, the cord and the copper alloy fibers were sections in three (one meter each one), in order to inspect surface and structural characteristics by means of microscopy and spectroscopy test respectively.
2.4.1 Topographic Test
After the initial inspection, we proceeded to develop topographic test survey microscope on the coating cable. We were searched for superficial changes such as, stress lines, pores, grooves, discoloration and deterioration of the material surface.
2.4.2 Spectroscopy (FTIR)
For compositional properties was performed an analysis by means of IR Spectroscopy. In this test we were searching for structural changes through R0 to R4 spectra.
2.3.4 Analytical Test and Final Disposal
The data were analyzed with Stat Graphics Centurion XVI software, employing the ANOVA test, the confidence of the study was placed in 99% (p=0.01) and logistic regression was employed for qualitative data of microscopy test. Residual samples were discarded as biological samples.
III. RESULTS AND DISCUSSION
3.1 Topographic Test
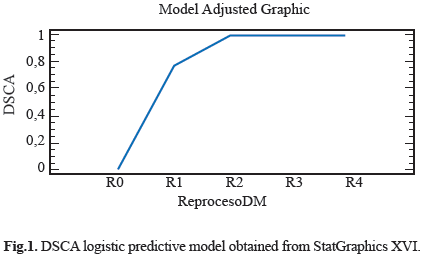
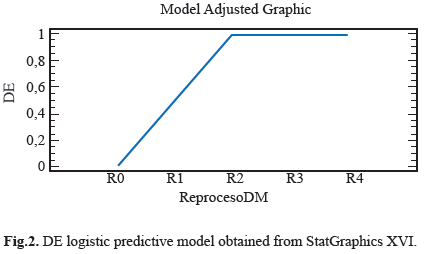
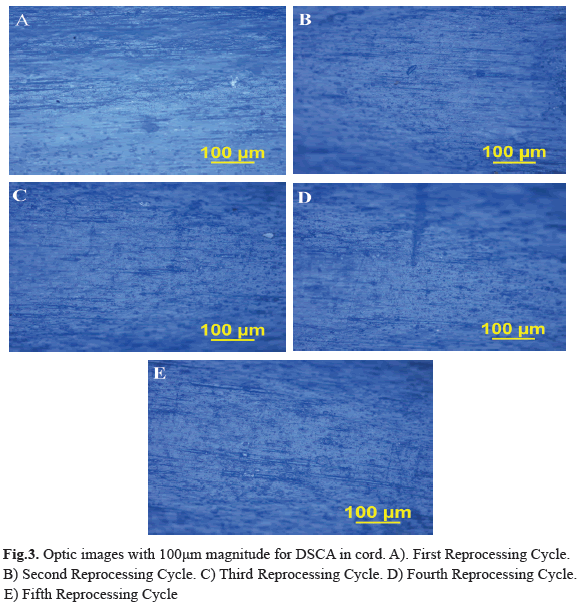
Different features were observed in the electro surgery cable surface, the Stain on Cable Insulator (MCA), Fluid or Residue on Cable Insulator (FORSCA) and Surface Defect on Cable Insulator (DSCA). For metallic elements we were searching for Corrosion on the Metal (CM), Defects in the Electrode (DE) and Defects in Copper Steely (DCA) as well. The results for each device in four reprocessed treatment are show in table 1. Representative relation between the reprocessing cycles, FORSCA and DE were found, the experimental shown how even from the reuse one (R1), the probability of default is very high 80%, before the reuse two (R2) the probability of found a surface defect in the cable is higher than above reuses, indicating that the forward treatments will be the presence of surface defects (Fig. 1) and (Fig. 2). For DSCA and DE we observed how the probability of finding defects on the electrode is higher than R0 sample, even from the first use (R1) we noted a change on the surface of stainless steel, which shown a predominance of abrasive lines (Fig. 3) and (Fig. 4) respectively. Since R1 both characteristics, DSCA and DE, shown the predisposition to present these effects, as well as describe in the Table 2.
3.2 Spectroscopy Test
With the intention of compare changes through R1 to R4, we found a reference spectrum [8].
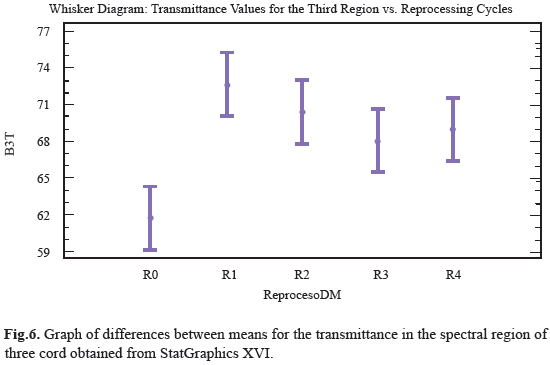
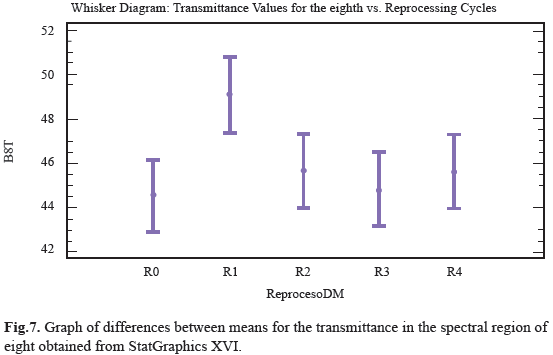
Ten distinct regions are observed with a specific functional group; these were numbered from 4000cm-1 to 600cm-1 and compared with the spectrum obtained from one pencil of the sample (Fig. 5). For the ten regions only the third and eighth region shown notorious changes in transmittance analysis, it can be seen how the range value for transmittance in this region was between 59% and 75%. The position of the functional groups was altered with respect to control sample after reprocessing cycles, the transmittance peaks decreased from R1 to R4 with as the reuse treatments advanced. This behavior was quantitatively analyzed to determine if there were differences between populations measures and showed that peak transmittance for the third region underwent significant changes due reuse processes, which indicates that the treatments are enough effective to affect the structure of polymer chain, although, unlike the others regions, these changes were more marked (Fig.6). For the eighth region can be observed that the range of values for the transmittance is 42% to 51%; is observed that R0, R2, R3, R4 did not underwent large variations in transmittance peaks including the last three tend to behave uniformly, as the same in past cases, we observe that R1 peak transmittance slightly increased in compare with control and other uses. This behavior was quantitatively analyzed to determine if there were differences between populations measures and were showed that transmittance peak for the region experiment some significant changes produced by reprocessing activities, this indicate that the treatments are affecting the structure of the chain region polymer (Fig.7). The results of the general spectroscopy test are shown in Table 3.
IV. CONCLUSIONS
According to the results, it can be inferred that the conditions associated with four reprocessing cycles with ethylene oxide generates small changes in the cord and more notorious for copper fibbers. It was proved that a small sample of 21 electrosurgical pencils is appropriated to make a quantitative analysis on differences in optical and spectral properties according to QUALY procedures.
We observed how surface defects in the cable insulation (DSCA) and defects in the electrode (DE), appeared as the advanced reprocessing cycles; for the other four conditions evaluated, heterogeneous behavior was observed, however, this was not uniform for all specimens, these defects are mostly abrasive. In fourth reprocessing we detect some defects, such as, surface staining fluid and spots, however they are not statically significant, these surface defects describe that the deterioration begins to appear on the material only for the fourth reprocessing.
For all regions evaluated in the spectrum was not found a change, with exception in the third and eighth region. By other hand, the transmittance peaks increase, and all of the samples followed the same pattern in advancing cycles. This means that the appearance of surface defects as previously observed in microscopy were due by structural movement in the polymer chain. There were no positional changes in the functional groups due reprocessing activities which means than this polymer sustain its structural composition.
Finally we recommended that the device could be reused four times without overall risk for the patient.
ACKNOWLEDGMENTS
We gratefully acknowledge reuse process support and the donation of devices from IQ Interquirofanos Aesthetic Clinic. We acknowledge financial support from the program of Biomedical Engineering from Antioquia School of Engineering - CES University and the economical support by the Young Researcher Program of Colciencias.
REFERENCES
[1]. Torres S., Montoya Y., Echeverri C. QUALY: reprocessing materials quality evaluation. Antioquia School of Engineering-CES University. Colombia. Radicated expedient patent nº 282757, 2013. [ Links ]
[2]. University of Otago, P. Day. What is the evidence on the safety and effectiveness of the reuse of medical devices labelled as single-use only?. Consulted on 2012 in http://enfermeriajw.cl/pdf/REUSE MEDICAL DEVICES.pdf. [ Links ]
[3]. Batista S., Uchikawa K., Padoveze M., Yaeko J. The sterilization Efficacy of Reprocessed Single Use Diathermy Pencils. Latino-Am. Emfermagem, 18(1), 81-6, 2010. [ Links ]
[4]. Howlin R., Harrison J., Secker T., Keevil C. Acquisition of Proteinaceous Contamination Through the Handling of Surgical Instruments by Hospital Staff in Sterile Service Department. Journal of Infection Prevention, 10(3), 106-111, 2009. [ Links ]
[5]. Lipscomb I., Sihota A., Keevil C. Diathermy Pencils: Reservoir For Proteins and Prion Contamination?. Journal of Hospital Infection, 64(2), 193-4, 2006. [ Links ]
[6]. Botero C., Uchikawa K., Andreoli T. Evaluation of Single-use Reprocessed Laparoscopic Instrument Sterilization. Latino-Am. Emfermagem, 19(2), 1-8, 2011. [ Links ]
[7]. Abramson S., Harold A., Best S., Bokros J., Brunski J., Colas A. Classes of Materials Used In Medecine. In: Ratner B, Hoffman A, Schoen F, Lemons J, Biomaterial Science: And Introduction to Materials in Medicine. Seattle: Elsevier Academic Press, 2004. 78-9 (Second edition). [ Links ]
[8]. Ismail H., Supri A., Yusof M. Blend of Waste poly(vinylchloride) (PVCw)/Acrylonitrile Butadiene-Rubber (NBR): the Effect of Maleic Anhydride (MAH) Blend of Waste Poly(Vinylchloride) (PVCw)/Acrylonitrile Butadiene-Rubber (NBR): the Effect of Maleic Anhydride (MAH). Polymer Testing, 23(6), 675-683, 2004. [ Links ]
[9]. Brunski, J. Metals. In: Ratner B, Hoffman A, Schoen F, Lemons J, editors. Biomaterials: An Introduction to Materials in Medicine. Seattle: Elsevier Academic Press, 2004. 141-3 (Second edition). [ Links ]
[10]. Spelman F. Cochlear Proteses. In: Ratner B, Hoffman A, Schoen F, Lemons J, editors. Biomaterials: An introdution to Materials in Medecine. Seattle: Elsevier Academic Press, 2004. 663-4 (Second edition). [ Links ]
[11]. Tessarolo F. Critical Issues in Reprocessing Single-Use Medical Devices. Causa International Symposium, 1, 1-35, Eindhoven, Netherlands, 2012. [ Links ]
[12]. Torres S., Paredes P., Montoya Y. Evaluating the properties of silicone hoses for aesthetic surgery subjected to cycles of reuse. IEEE Xplore Digital Library, 1, 1-6, 2011. [ Links ]
[13]. ValleyLab: Surgical Products Catalogue. Online, 2004. Available in URL: http://paginasprodigy.com/tmx8183005508/Catalogo%20Valleylab.pdf. [ Links ]