Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Ingeniería Biomédica
Print version ISSN 1909-9762
Rev. ing. biomed. vol.10 no.20 Medellín July/Dec. 2016
METHOD OF STRATEGIC INCORPORATION OF BIOMEDICAL TECHNOLOGY FOR HEALTH INSTITUTIONS
MÉTODO DE INCORPORACIÓN ESTRATÉGICA DE TECNOLOGÍA BIOMÉDICA PARA INSTITUCIONES DE SALUD
MÉTODO DE INCORPORAÇÃO ESTRATÉGICA DE TECNOLOGIA BIOMÉDICA PARA INSTITUIÇÕES DE SAÚDE
M.A. Ardila1, A. Gómez1, J.E Camacho-Cogollo2
1 Estudiante ingeniería biomédica, Universidad EIA, Medellín, Colombia.
2 Docente Investigador, Universidad EIA, Medellín, Colombia. Author's mailing address: Javier.camacho@eia.edu.co.
ABSTRACT
The continued high and unnecessary costs of technology, poor patient care, decisions made by people with little experience and waste of public resources devoted to health. All generate a need to develop a rational and systematic process for the incorporation of medical equipment. The aim of this work was to create such a method through collection of information, a literature review and implementation of a survey to diagnose the status of the processes of incorporation into different healthcare institutions and learn the critical stages and steps to be performed in each. The method was implemented in a real case with the acquisition of two technologies, a linear accelerator and a steam sterilizer. The method was validated with two experts responsible for the acquisition of this equipment to determine its usability and importance in the process of incorporation of biomedical technology. Validation showed quantitative and positive results for both technologies because the experts were satisfied with each of the aspects evaluated and the final report provided by the method.
KEYWORDS: Biomedical technology; Incorporation; Technology assessment; Acquisition.
RESUMEN
Los costos continuos, altos e innecesarios de la tecnología, la escasa atención del paciente, las decisiones tomadas por personas con poca experiencia y el desperdicio de recursos públicos dedicados a la salud, generan la necesidad de desarrollar un proceso racional y sistemático para la incorporación de equipos médicos. El objetivo de este trabajo fue hacer que un método comience con una recopilación de información a través de una revisión de la literatura y la implementación de una encuesta para diagnosticar el estado de los procesos de incorporación en diferentes instituciones de salud y saber cuáles son las etapas críticas y los pasos a seguir en cada uno de ellos. El método se implementó en un caso real con la adquisición de dos tecnologías: el acelerador lineal y un esterilizador de vapor. El método fue validado con dos expertos responsables de la adquisición de estos equipos para determinar su usabilidad e importancia en el proceso de incorporación de la tecnología biomédica. La validación mostró resultados cuantitativos y positivos para ambas tecnologías, ya que los expertos estuvieron satisfechos con cada uno de los aspectos evaluados y el informe final proporcionado por el método.
PALABRAS CLAVE: Tecnología biomédica; incorporación; tecnología; adquisición.
RESUMO
Os altos e desnecessários custos continuados da tecnologia, a escassa atenção aos pacientes, as decisões tomadas por pessoas com pouca experiência e o desperdício de recursos públicos dedicados à saúde, geram a necessidade de desenvolver um processo racional e sistemáticos para a incorporação de equipamentos médicos. Os objetivos deste trabalho foram fazer um método que comece com uma compilação de informação através de uma revisão da literatura e a implementação de uma sondagem para diagnosticar o estado dos processos de incorporação nas diferentes instituições de saúde e saber quais são as etapas críticas e os passos a seguir em cada um deles. O método implementou-se num caso real com a aquisição de duas tecnologias, o acelerador linear e um esterilizador de vapor. Este foi validado com dois peritos responsáveis da aquisição destes equipamentos para determinar sua usabilidade e importância no processo de incorporação da tecnologia biomédica. A validação mostrou resultados quantitativos e positivos para ambas tecnologias, já que os peritos estiveram satisfeitos com cada um dos aspectos avaliados e o relatório final proporcionado pelo método.
PALAVRAS-CHAVE: Tecnologia biomédica; Incorporação, Auditoria tecnológica; Aquisição.
I. INTRODUCTION
The high level of development of biomedical devices turns health centers into technologically advanced spaces, which then turns medical equipment into indispensable tools for solving human health problems. It is estimated that health care will depend on the development, availability and acquisition of new technologies by way of its proper evaluation and management. The incorporation of technology (IT) is part of the macro process of technology management, which includes maintaining the sub-processes of adequate planning and technology acquisition to avoid unnecessary expenses that put the health-care programming and finances of health institutions at risk [1-5].
The evaluation of technology, which is the first stage of the process of incorporating technology, is a process that requires the work of experts in various disciplines to synthesize information regarding medical, social, economic and ethical issues related to the use of health technology in a systematic, transparent, impartial and robust manner [4].
The method proposed in this paper seeks to quantify the results of the processes performed in the incorporation of IT by designing formats that take into account important aspects such as safety, clinical benefits, economics, ethical and social needs and services from suppliers [4, 7]. The weight in percentage is assigned to each criterion to make a decision based on the numerical results and thus adjust the available budget of each institution and direct all its actions to the fulfillment of the projected future goals [8].
II. METHODOLOGY
The study was developed in four phases: the first consists of a bibliographical review of academic works, followed by the performance of a comparison of the methods of incorporation in a global context. The results provided the basis for the construction of a survey of different clinical engineers in Colombia, which allowed for the establishment of the necessary steps for the elaboration of a methodological proposal. In the next phase the proposal was constructed describing the associated formats and guide for their use. In the third phase, the method is implemented in two cases of medical equipment incorporation, and its possible impact is measured through two expert evaluations. Finally, the results obtained are analyzed and the degrees of usability, importance and impact of the proposed method in saving resources for health institutions is determined.
III. RESULTS
The results of this development will be presented in the sequence of phases as established in the methodology.
A. Analysis of the survey
For the development of the survey, the Qualtrics survey design tool was used, which generates a graphical analysis of the results. The survey was sent to 25 clinical engineers (CI) from Colombia's Service Providing Institutions (in Spanish, Instituciones Prestadoras de Servicios or IPSs).
The survey provided results that were used to generate a diagnosis on the implementation of strategic technology incorporation in the country. Of the sample selected, only 68% of health institutions have a multidisciplinary committee structured as part of the IT process, and only 26% of professionals are satisfied with the process. Likewise, a flaw in prioritization was detected in IT, since bad practices were evidenced in the identification of needs, and generally obsolete equipment or those with a history of failures are not replaced. This is complemented by the fact that 47% of the IPSs do not have an IT plan with projection over time, generating the perception of little strategic planning for purchase plans and economic budgets.
On the other hand, the CIs indicated that the most relevant considerations for an adequate IT process should include technology technicians as aspects, in addition to variables such as patient safety, infrastructure adjustments, clinical benefits, pathologies to be treated, and lastly ethical/social aspects. Fig. 2 illustrates the levels of importance as CIs assigned them during the survey.
For the IC professionals, the most important aspects to evaluate in a biomedical technology supplier are technical capacity and availability of spare parts and accessories, accompanied by the warranty periods offered. Also considered were quality assessments, product delivery times, payment methods, references from other institutions, and experience in the market. Other crucial aspects for the IPS were the warranty conditions as well as response times to calls for technical service requests, and that the product does not have alert reports at the national or international level.
Aspects such as compatibility with other equipment in the institution, support with training, opinions of future users and ease of cleaning and disinfection are factors also taken into account.
B. Construction of the Method
Fig. 1 illustrates the method developed. The sequence of the method is described through a flowchart, providing a general idea of the steps that must be followed and to allow for the organized development of the proposed method.
The proposed method was designed based on the considerations expressed by the CIs in the survey, in addition to respecting the concepts of planning, acquisition and management, and allowing for articulation with the policies and strategic plans of the health institution that wishes to implement it [4, 5].
To apply the method correctly, it is important that the institution be tertiary care and have the following characteristics:
- A needs committee that performs internal and external analysis [5].
- Software that allows for inventory storage of institution biomedical equipment with the resumes, maintenance history and spare parts used, operating status, and history of adverse events.
- An evaluation committee with the powers to receive, analyze and evaluate requests for technology in all health-care departments [6].
- An acquisition committee in charge of carrying out the purchase, reception, installation, implementation and monitoring processes of the technology.
Depending on the size and number of the institution's medical teams, the needs committee should be composed of two biomedical engineers, one in charge of identifying the internal needs of the institution and another to identify the external needs. It is recommended that this person be dedicated exclusively to these tasks [5].
The committee should meet regularly to evaluate new and emerging technologies already on the market for the identification of future acquisitions. In addition, they should analyze which equipment needs to be renewed or updated. When performing the self-diagnosis, they must take into account both the technological situation and the signals received from the environment through technological vigilance [7].
The evaluation committee is a temporary association during the incorporation process, a multidisciplinary group that represents different perspectives and performs an analysis of the evidence and useful recommendations for the entire hospital structure [8]. It is recommended that this committee be made up of a CI, a finance representative, and an architect if necessary. In addition to this, the opinions of the healthcare staff, a doctor of the specialty where the technology is going to be required and/or a head nurse in the department and personnel who will manage the equipment should be sought [9].
The CI within his or her functions in the committee should be responsible for the facilities management: impact on the environment and public services requirements; information technology: support software and network issues; materials management: supplies, accessories, alternative vendors, equipment and auxiliary furnishings, alternative sources of supplies and services; health information: epidemiological data and regulations; manufacturing: product specifications (technical data sheet), installation, operational requirements and warranty [8].
The CI must determine the true need for technology acquisition, analyze special safety and performance considerations, select the product to be evaluated, write the purchasing specifications, direct the product evaluation, select the final product, and conduct the performance evaluation of the product once it is implemented [6].
The method makes it possible to identify both the external and internal needs of the institution, prioritize acquisition requests, determine what kind of technologies are feasible to acquire based on the results of the evaluation, and suggest which supplier, make and model are the most suitable according to the technical specifications, costs and services that each offers.
The proposed method is structured on the basis of a continuous IT process linking five (5) types of assessments which are described below:
Needs Assessment: This first process begins with the implementation of a culture of reporting of real needs by the health-care services, always differentiating needs and desires, avoiding at the outset the possibility of acquiring equipment that could be underused in the future or cost overruns on the purchase of accessories and consumables [2].
The process includes the concept of technology monitoring as a source of external needs assessment, identifying new and emerging technologies in the market to plan future acquisitions based on the health services they wish to provide [3]. These external needs are articulated with the epidemiological trends reported by national and regional organizations, plus they allow for the analysis of the institution's technological capabilities based on the results of an internal needs assessment. An assessment inventory must be made of the equipment to identify the existing technologies in the institution, which have the possibility of being changed, how this change would take place and what it would cost, in this way a plan could be drawn up of what could be acquired in the short and medium term [5].
Safety Assessment: At this point, the method considers the risks and history of recalls associated with a piece of equipment before making a purchase decision [6].
This evaluation can have two approaches, the first only takes into account the imminent risk of a device according to its complexity and means of use, it considers the probability of failure during use. The second quantifies the probability that arises from past experiences, and is collected through a search of information in databases, agencies or institutions such as FDA, ECRI and INVIMA among other assessment and certification bodies [7].
Evaluation of Benefits: Here, the possible direct and indirect benefits are considered that the piece of equipment under consideration may bring to the institution. Typical examples of benefits are the reduction of deaths or illnesses, gains in life expectancy or quality of life, and increases in effectiveness, productivity (reduction of procedural times) or reliability [2].
During the evaluation, indirect benefits should not be ignored, which can help make the final decision to acquire or not acquire a piece of equipment. Some indirect benefits can be: increased user productivity due to the satisfaction of working with new technology or a better user interface (psychological factor), additional income to other procedures resulting from this new technology, more coverage and improvement in access. Depending on the relevance, the benefits of the impact on the brand or prestige of the institution should also be analyzed. Variables associated with this include marketing and competitiveness, such as recruiting recognized physicians and surgeons, market competitiveness or higher rates of earnings [3].
Financial Assessment: The financial benefits associated with the acquisition of new technologies are reflected in the increase in income (increase in productivity, increase in profit rates, etc.), decrease in expenses (less maintenance costs, lower probability of risk, shorter patient stay, and reductions compared to other institutions, etc.)[3, 4].
The financial evaluation considers solving questions regarding how much the institution is willing to invest to generate a differentiating health product versus the market offer, and how insurers will be willing to negotiate and pay for them. The method also suggests always carrying out a cost benefit analysis, comparing the different technological alternatives proposed by suppliers, identifying in this case all the possible benefits that, in economic terms, can impact the finances of the IPS [7].
The method proposes another important economic factor to be analyzed: the learning curve for users as a result of IT. The need for training physiotherapists, nurses, physicians, and others involved in the use of technology is a fundamental factor. These professionals often need to perform pre-use check-ups, emergency interventions in the event of equipment failure, and understand the limitations of the technology [3].
Ethical and Social Evaluation: this evaluation intends to establish what impact the IT and its use would have on society, therefore the method proposes contemplating ethical aspects that could influence the decision whether to incorporate new technology or not [7].
Legal provisions affecting the acquisition, accessibility of technology, compliance with human rights, human integrity and dignity, potential conflicts with religious and cultural convictions, and stigma and discrimination should therefore be studied [7].
Within the ethical evaluation of technology, it is important to take into account factors such as whether the signing of an informed consent for special situations is necessary when performing a surgical medical procedure.
Acquisition: After performing the different technology evaluation processes, the method proposes carrying out the acquisition stage, where it is articulated with the procedures established by the IPS, and where what is required is obtained according to the plan.
For this stage, the method proposes implementing socialization and participation sessions for professionals from different disciplines, for example, listening to the opinion of both the medical specialists who will make use of the technology and the biomedical engineers who have the perspective on how the technology will be used and operated, and the main aspects that must be taken into account for each type of technology [4].
The activities carried out during this stage must be constantly monitored and not relegated to a single area of the institution because there are many critical decisions that must be made during the acquisition, decisions requiring people with solid knowledge and a clear understanding of the mission and objectives to be achieved when carrying out the acquisition [3].
Selection: For the selection process, the method proposes to collect the same information from different suppliers in a specific format. The next step is to compare one product with another to identify the technology that best fits with the benefits and considerations generated in the previous evaluations [6].
Specifications are typically grouped into general categories that include overall performance, safety, userfriendliness, and other user-related factors, and build quality, service, and overall cost. The categories do not have equal weight on the final decision and there will be some that will require more attention than others [6].
When we come to the stage of comparing the costs of the different options being evaluated, all the costs of having the technology during its useful life must be considered so that the financial evaluation of each of the products is complete [6].
Because the cost after purchase is between 2-5 times the acquisition costs, the method calls attention to the importance of evaluating all costs before making a purchase [3].
Procurement process: This step includes the documents completed in the planning process, such as justification of the technology, the ETES, the result of the evaluation of the proposals and the strategic plan. The result of the evaluation of the proposals must clearly indicate all the services offered by the selected supplier in each of the technical characteristics such as maintenance, spare parts, etc.
In this phase, the method suggests drafting the contract and establishing the method and conditions for the equipment delivery. Once the contract is signed, the purchase is carried out. The supplier must be sent a list of the necessary documents which must be delivered when the equipment is received.
Receiving and installation: The technology is received, it is verified that the documents described are in order and that the equipment is in optimal condition. To determine this a functional test is performed. It is recommended that this receiving process be performed in the engineering area.
The equipment is transferred to the department, installation of the equipment in the department is carried out, and operation and safety tests are checked again. Once approved, the receipt of satisfaction is signed by the area manager.
Training: Trainings are conducted according to the schedule agreed upon in the request, both for engineering and healthcare staff, ensuring a complete understanding of the use of the technology. All personnel that will be in charge of the use of the technology must be satisfied with the information provided. All training must be carried out before the equipment is implemented [12].
Implementation and follow-up: The department is authorized to use the equipment when required. It is recommended to monitor operation to ensure it is in optimum condition.
Follow-up on the technology is done once it is installed and implemented in order to verify that it meets the expected demand and technical characteristics offered, whether it fulfills the needs and generates the benefits that were identified in the evaluation of the acquisition.
This step should be done by defining and monitoring indicators that should define the institution according to their needs and the information they wish to obtain from this follow-up. From this stage, feedback should be generated for the overall IT process as well as for the adjustment of the institution's strategic plans and budgets.
C. Application and validation
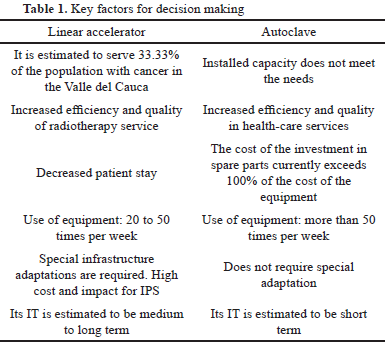
For this point, the method was applied in an IT case study of two pieces of medical equipment in an IPS in the city of Cali, Colombia. The IPS intended to acquire one piece of equipment for technological renovation and another considered new. The validation was done through the consultation of two experts of the selected IPS, the variables of usability and quality of information that the method provides for decision making were considered.
- Case study
The validation was carried out at the Clínica de Occidente in Cali, the pieces of equipment selected were: a linear accelerator as a new technology and an autoclave as a renovation.
The method helped identify key factors for decision making, which are summarized in Table 1.
The whole process of designed incorporation was carried out for each of the devices. The corresponding forms were filled out, each of the stages were evaluated based on scores and in the end the most suitable supplier was defined according to the technical specifications and the conditions offered by the different suppliers evaluated.
D. Validation with experts
The results obtained from the implementation of the method were evaluated by two experts from the Clínica de Occidente through the processing of a question form. The two experts were the coordinator of the engineering area, in charge of managing the institution's technology, and the operational manager whose role is to manage high-cost technologies, such as the linear accelerator.
The validation of the method was successful, had good approval by the experts, and they considered it well structured and a great help for effective and quality decision making.
In general, the results exceeded the initial expectations, the experts expressed their satisfaction with the process that the method describes, the way in which the results are presented, and the reports for each of the technologies. There is a desire to use the information provided by the method to make the final decision and enthusiasm for the help represented by this tool in the biomedical IT processes in the IPS.
IV. DISCUSSION
A variety of IT-related articles were found that formulate the most important aspects that should be evaluated before acquiring a technology. However, most of the methods found in the literature are very general and yield qualitative results, which is why the proposed method generates added value by generating quantitative data that allows for the structuring of more accurate decision making.
The analysis of invisible costs is a critical aspect when evaluating a piece of equipment and is usually not considered at the time of purchase. There is evidence that invisible costs are even greater than the value of the equipment, and it is possible that the institution may not be able to cover these costs over the useful life of the technology.
The viability of the method was verified through its application in the process of incorporating a linear accelerator and an autoclave at the Clínica de Occidente. It was confirmed that the method presents an adequate system to identify both the internal and external needs of the institution, helps to prioritize procurement requests, determines what kind of technologies are feasible to acquire, and based on the results of the evaluation, suggests which supplier, brand and model are the most suitable according to the technical specifications, costs and services that each offer.
The application of the method gave the conclusion that warranty time is a factor of high weight in the total cost of the useful life of the equipment. This is due to the fact that no spare parts and maintenance costs are generated during the years of the warranty. Based on this, it is recommended that the Clínica de Occidente, in the case of acquiring the linear accelerator, request an extension of the period of time when the warranty is in effect, as it is a high investment technology in the short and long term.
It was identified that the purchase of a piece of equipment can not be based on its price because the selected technology may not be the cheapest but may offer better specifications or services after sale, as was shown in the case of the linear accelerator, where the selected technology is more expensive but offers services that make it a better choice than that from the other provider.
The validation with the experts concludes that although it is a well-structured method for the proper incorporation of technology, it does not achieve excellence and should improve in aspects like clarity in content and quantity of evaluated criteria so that its application in health institutions is more feasible.
In general, they consider that it is a tool that helps decision making because it helps to clear up doubts and compare and select the best option. Both experts expressed an interest in using the information generated by the tool to make their final acquisition decisions.
V. CONCLUSIONS
The developed method guides the user to carry out the entire process of incorporation in a simple and systematic way.
It helps the institutions to identify needs by evaluating the state of the technology they have and the most relevant medical conditions in the region, and then, based on the policies and goals that the institution wishes to achieve, to decide what technology is most appropriate to meet those needs.
It proposes a standardized process for making objective decisions and helping with the organization of processes and procedures of the institution, which contributes to the fulfillment of quality standards, which is an essential subject in a tertiary care IPS.
For the application of the method, the institution must be of tertiary care and have the software to manage the inventory and the committees for needs, evaluation and acquisition. This is because the method was developed assuming that the institution had these characteristics, and if the institution does not have them, application of the method will be difficult. The method is valid to strategically incorporate biomedical technology in a health institution because it suggests what decision to make at each stage of the process. Proof of this is the expert verification and feedback upon validating the method and tool.
The objectives were fulfilled satisfactorily and were exceeded when applying the method in a real case of incorporation analysis of two biomedical technologies in the Clínica de Occidente in the city of Cali. It was possible to use the formats both for the incorporation of a new technology as with the linear accelerator, and for incorporating existing technologies in the institution as with the autoclave.
REFERENCES
[1]. Castañeda C., Fonseca M., Núñez J., Zapata J.; Gonzalo J. La Sostenibilidad Financiera del Sistema de Salud Colombiano: Dinámica del gasto y principales retos de cara al futuro, Bogotá: Fedesarrollo, 2012. [ Links ]
[2]. Organización Mundial de la Salud. Resolución WHA60.29. (julio de 2007). Consultado el 15 de noviembre de 2016 en: http://www.who.int/medical_devices/policies/resolution_wha60_r29-sp.pdf. [ Links ]
[3]. Wang B. Strategic Health Technology Incorporation, Organ & Claypool, 2009. [ Links ]
[4]. Aparecido Nunes A., Marques de Mello L., Wichert Ana L., Mazzoncini de Azevedo Marques P., Lessa Dallora M. E., Zangiacomi Martinez E., Pazin Filho A., Barbosa Coelho E. Avaliação e incorporação de tecnologias em saúde: processo e metodologia adotados por um hospital universitário de alta complexidade assistencial. Cadernos de Saúde Pública, 29(1), 179-186, 2013. [ Links ]
[5]. Santos F.A., Margotti A.E., Ferreira F.B., Garcia R. Health Technology Assessment Applied to Health Technology Management through Clinical Engineering. IFMBE Proceedings, 41, 1104-1107, 2013. [ Links ]
[6]. Organización Mundial de la Salud. Guía de recursos para el proceso de adquisición, Ginebra: OMS, 2012. [ Links ]
[7]. Paz F.A., Del Valle Made E., Hadad Salomón R. Ethical and legal model for technological surveillance system. Interactive Collaborative Learning (ICL), 999-1006, 2015. [ Links ]
[8]. Carvajal Tejada M.; Ruiz Ibáñez C.G. Evaluación técnica y clínica de tecnología biomédica en procesos de adquisición: un enfoque en evaluación de tecnologías en salud. Revista Ingeniería Biomédica, 2(4), 34-45, 2008. [ Links ]
[9]. Calil S. J. Caminhos para a incorporação de tecnologias em saúde. Revista Debates GV saúde, 3, 31-34, 2007. [ Links ]
[10]. Atles L. A practicum for Biomedical Engineering & Technology Management Issues, Kendall/Hunt Publishing Company, 2008. [ Links ]
[11]. Gonzáles D. Herramienta de evaluación para adquisición de equipos biomédicos. Medellín. 2014. [ Links ]
[12]. Van Hove P., van Wijk R., Verdaasdonk E., Stassen L., Dankelman J. Training and assessment of equipment-related competence. Comparison of a petrochemical company and a hospital. Health technology, 3, 221-226, 2013. [ Links ]











 text in
text in