Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Universitas Medica
versão impressa ISSN 0041-9095versão On-line ISSN 2011-0839
Univ. Med. vol.60 no.4 Bogotá out./dez. 2019
https://doi.org/10.11144/javeriana.umed60-4.crna
Research articles
Characterization of Newborns with Moderate or Severe Perinatal Asphyxia Managed with Selective Cerebral Hypothermia in the Newborn Unit of the Hospital Universitario San Ignacio from June 2015 to March 2017
1Unidad de Cuidado Intensivo Neonatal, Departamento de Pediatría, Hospital Universitario San Ignacio. Profesora del Departamento de Pediatría de la Facultad de Medicina, Pontificia Universidad Javeriana, Bogotá, Colombia
2Unidad de Cuidado Intensivo Neonatal, Departamento de Pediatría, Hospital Universitario San Ignacio. Profesora ad honorem, Facultad de Medicina, Pontificia Universidad Javeriana, Bogotá, Colombia
3Unidad de Cuidado Intensivo Neonatal, Departamento de Pediatría, Hospital Universitario San Ignacio. Directora del Departamento de Pediatría, Facultad de Medicina, Pontificia Universidad Javeriana, Bogotá, Colombia.
4Servicio de Genética, Hospital Universitario San Ignacio. Instituto de Genética Humana, Facultad de Medicina, Pontificia Universidad Javeriana, Bogotá, Colombia. Director del Instituto de Genética Humana, Facultad de Medicina, Pontificia Universidad Javeriana, Bogotá, Colombia.
Introduction:
Hypoxic- ischemic encephalopathy generates various alterations in the central nervous system of the newborn, with various consequences ranging from physical and mental disability to death. The evidence indicates that moderate to severe hypoxic-ischemic encephalopathy must be managed with therapeutic hypothermia.
Objectives:
Describe the clinical characteristics of a group of neonates treated by the therapeutic hypothermia team of the Neonatal Intensive Care Unit of the Hospital Universitario San Ignacio.
Methodology:
Sequential cross-section observational study, from June 2015 to March 2017. Continuous variables were compared using student's t-test. Relative frequency analysis was performed and compared according to variants of clinical interest using chi-square.
Results:
A total of 41 patients were treated by the program during the observation period. Clinical seizures were evident in 65% of the subjects. The main clinical outcome to discharge observed in the patients after the intervention was encephalopathy (90%). The clinical determinants of the birth of the neonate are compared with the death outcome where no significant differences were found proportionally between the two groups except for Apgar at 10 minutes and the presence of acidemia. For the outcome of death, RP greater than 1 in the case of Cesarean section, increased clotting time, thrombocytopenia, hypocalcemia and impaired renal function (p <0.05 and IC-95> 1).
Conclusions:
Low Apgar at 10 minutes and acidemia is associated with higher mortality. There was a greater prevalence of electrolyte and renal coagulation alterations in the group with lethal outcome. The time of onset of the therapy was related to seizures but not to death.
Keywords hypoxia; newborn; hypothermia; infant; diseases; perinatal mortality
Introducción:
La encefalopatía hipóxico-isquémica altera el sistema nervioso central del recién nacido, con consecuencias que incluyen desde discapacidad física y mental hasta muerte. La moderada a severa debe manejarse con hipotermia terapéutica.
Objetivo:
Describir las características clínicas de un grupo de neonatos con encefalopatía tratados con hipotermia terapéutica en la Unidad de Cuidado Intensivo Neonatal del Hospital Universitario San Ignacio.
Metodología:
Estudio observacional de corte trasversal secuencial, desde junio de 2015 hasta marzo de 2017. Se compararon las variables continúas utilizando la T de Student. Se analizaron las frecuencias relativas y se compararon según variantes de interés clínico utilizando chi cuadrado.
Resultados:
El programa atendió 41 pacientes durante el periodo de observación. se evidenciaron convulsiones clínicas en el 65 % de los sujetos. El principal desenlace clínico observado al egreso luego de la intervención fue algún grado de encefalopatía (90 %). Los determinantes clínicos del nacimiento del neonato se comparan con el desenlace muerte, donde no se encontraron diferencias significativas entre ambos grupos, a excepción de Apgar a los 10 minutos y acidemia. Para el desenlace muerte, RP mayores a 1 en el caso de cesárea, incremento de tiempo de coagulación, trombocitopenia, hipocalcemia y alteración de la función renal (p < 0,05 e IC-95 %: >1).
Conclusiones:
El Apgar bajo a los 10 minutos y la acidemia presentaron mayor mortalidad. Hubo mayor prevalencia de alteraciones de coagulación, electrolíticas y renales en el grupo con desenlace letal. El tiempo de inicio de la terapia se relacionó con convulsiones, mas no con muerte. Palabras clave hipoxia; recién nacido; hipotermia; enfermedades del recién nacido; mortalidad perinatal.
Palabras clave hipoxia; recién nacido; hipotermia; enfermedades del recién nacido; mortalidad perinatal
Introduction
Perinatal asphyxia is one of the leading causes of mortality in children under the age of five years (1,2). It is estimated that for each patient who dies due to perinatal asphyxia there are 10 survivors with serious sequelae (3). In the Bogotá Capital District, for 2014, perinatal asphyxia represented 6.4% of early neonatal mortality (4). Hypoxic-ischemic encephalopathy (HIE) is the set of alterations in the central nervous system of the newborn, caused by oxygen deprivation and the subsequent ischemia. This clinical picture was originally described and classified by Sarnat and Sarnat (5,6).
Since 2010, the American Heart Association and the International Liaison Committee on Resuscitation have recommended that all newborns older than 36 weeks of gestational age with moderate-to-severe HIE should be offered the possibility of therapeutic hypothermia within the post-resucitation care. This recommendation was endorsed in the Clinical practice guide for newborns with perinatal asphyxia, published in April 2013 by the Ministry of Health and Social Protection of Colombia (7,8).
Current findings have shown that the use of therapeutic hypothermia within the first six hours after the asphyxia event reduces statistically and clinically the combined result of mortality or disability longer than 18 months, without increasing the risk of disability among survivors and with positive effects that remain until childhood (9,10).
The program of Selective Hypothermia with Cool-Cap (helmet) in the Neonatal Intensive Care Unit of Hospital Universitario San Ignacio offers this therapeutic option to newborns with HIE. The present article describes the experience of the treating team with a group of neonates treated at the hospital. This contributes to the knowledge of this area of newborn care, by presenting clinical outcomes of interest in the context of a tertiary hospital in Colombia.
In Colombia, a cohort of patients treated with total body therapeutic hypothermia during 2017 has already been described (11). The study aimed to describe the results obtained with the other method of selective hypothermia: blanket.
Methods
Observational, sequential cross-sectional study. Patients’ data include an observation from June 2015 to March 2017. The sample size was based on the census and included all patients referred to the program during the observation period with asphyxia criteria, defined by the American Academy of Pediatrics and the American College of Gynecology and Obstetrics as metabolic or mixed acidosis (pH < 7.0 in a sample of cord blood at birth), 5-minute Apgar score < 3, neurological involvement (seizures, coma and hypotonia) and involvement of multiple organs (heart, lung, liver, kidney and intestines) (6).
Selective cerebral hypothermia therapy with Cool-Cap is used to treat newborns with moderate to severe HIE, in order to selectively cool the head with moderate systemic hypothermia (34-35 ºC), reduce mortality and prevent or reduce the severity of neurologic injury associated with HIE. Patients remain in intensive care with continuous monitoring of vital signs and brain activity by means of an extended electroencephalogram. The therapy lasts 72 hours, and then reheating is carried out at 0.5 ºC per hour until reaching physiological temperatures (36.5-37.5 ºC).
The variables studied were defined according to what is reported in the literature (5,12) and to the care protocol of the newborn unit. The variables were collected directly from the patients’ medical records, tabulated in an Excel® 2010 spreadsheet, and exported to the Stata 14 software for analysis. The presence of encephalopathy at discharge and death were considered outcome variables. The graphs were generated with GraphPad 7.04.
Continuous variables were compared using the Student’s T test. Relative frequencies were analyzed and compared according to variants of clinical interest using chi square. The prevalence ratio and their 95% confidence intervals (95% CI) were also calculated for the encephalopathy and death outcomes according to the clinical variables of interest. An α = 0.05 (significant p <0.05) was considered for all the statistical data used. For categorical variables such as Apgar, 3 groups were established: 0-5, 6-7 and 8-10, and were compared using Kruskal-Wallis.
Variables were defined as follows: severe arrhythmia (different from sinus bradycardia or bigeminy), severe hypotension (when, despite volume administration, dopamine was required at more than 20 μg/kg/minute) and venous thrombosis (vessel thrombosis not related to venous access lines). For the other variables defined as postnatal complications we took a time of 7 days of life, and we considered:
Mild hypotension: average blood pressure < 40 mm Hg.
Coagulopathy: clinical bleeding or alteration of clotting times.
Renal failure: urinary output < 0.5 ml/kg/hour for more than 24 hours or increase in creatinine higher than 009 mmol/L.
Hyponatremia: seric sodium < 135 mmol/L.
Hypokalemia: seric potassium < 3.5 mmol/L.
Thrombocytopenia: platelets < 150,000 per uL.
Elevation of liver enzymes: AST > 200 IU/L and ALT > 100 IU/L.
Metabolic acidosis after starting the protocol: pH < 7.34 or base deficit ≥ 4 mmol/L.
Respiratory difficulty: need for mechanical ventilation or continuous positive airway pressure (CPAP).
Systemic infection: positive cultures in blood, cerebrospinal fluid or urine taken after starting therapy.
Hemoconcentration: increase in hematocrit > 20%.
Hypoglycemia: glycemia < 47m/dL.
Hypocalcemia: calcium < 8 mmol/L.
Difficulty maintaining the target temperature: less than 33.5 ºC or more than 37.5 ºC for more than one hour.
Ethical aspects
All data is reported in an aggregated and anonymous manner. The database is kept in the computers of the researchers with unique usernames and passwords and under the security parameters of the Pontificia Universidad Javeriana. The study protocol was reviewed and approved by the School of Medicine and Hospital Universitario San Ignacio Research Ethics Committee.
Results
41 patients were treated in the program during the observation period. The protocol admission criteria were: newborns ≥ 36 weeks gestational age and body weight ≥ 1800 grams who had moderate or severe encephalopathy defined by A and B criteria, indicated as:
Criteria A: One or more of the following findings: 10-minute Apgar score ≤5, continuous resuscitation including endotracheal intubation or mask 10 minutes after birth, umbilical cord blood pH < 7.0 in the first hour of life , excess base ≥ -16 in umbilical cord gases or in any blood sample in the first 60 minutes of life.
Criteria B: Altered state of consciousness (lethargy, stupor or coma), and at least one of the following: hypotonia, abnormal reflexes or pupillary abnormalities, weak or absent sucking reflex, clinical seizures.
If the patient met criteria A and B, an amplitude electroencephalogram was performed. Patients with imperforate anus and major congenital abnormalities were excluded. A patient with panhypopituitarism undiagnosed at birth was included.
Table 1 describes the general characteristics of the subjects when entering the program. 14% of the cases were between 36 and 37 weeks gestational age (GE), 82% of the cases were between 38 and 40 weeks GE, there was only one case with 41 weeks. The most frequent gestational age was 39 weeks (41.5%). The most frequent type of delivery was natural birth (51.2%). Regarding the electroencephalographic (EEG) activity before the intervention, 61% of the cases suffered from abnormal activity of moderate type and 17% had abnormal EEG activity of severe type.
Table 1 General characteristics of the patients served by the program
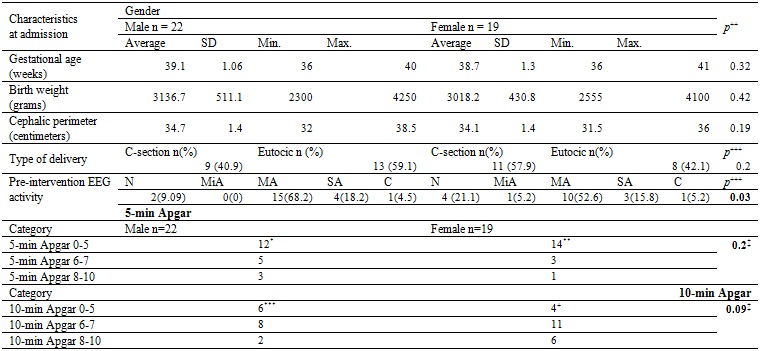
The main characteristics defining the delivery are presented discriminated by sex. No significant differences were found in the proportion of cases by sex related to gestational age, birth weight, cephalic perimeter, Apgar, or type of delivery. Abnormal electroencephalographic activity of moderate type predominated and was present in 61% of cases. *Based on 20 observations. **Based on 18 observations ***Based on 19 observations +Based on 17 observations. SD: Standard deviation. N: normal. MiA: mild abnormal. MA: moderate abnormal. SA: severe abnormal. C: convulsive. +Mean differences with equal variances. +++Difference of proportions. ‡Kruskal-Wallis.
The treatment was performed for 72 hours. Table 2 shows the main postnatal complications. Clinical seizures were evident in 65% of the subjects, and respiratory difficulty in 48% of the subjects. The most frequent electrolyte disorders were hyponatremia, in 56% of cases; hypokalemia, in 31%, and hypocalcemia, in 26% of patients. 29% of the patients presented pulmonary hypertension, and 26% presented clotting disorders. Bradycardia is commonly observed during therapy, without hemodynamic repercussion. There were no cases of venous thrombosis or hemoconcentration.
Table 2 Adverse events during therapy
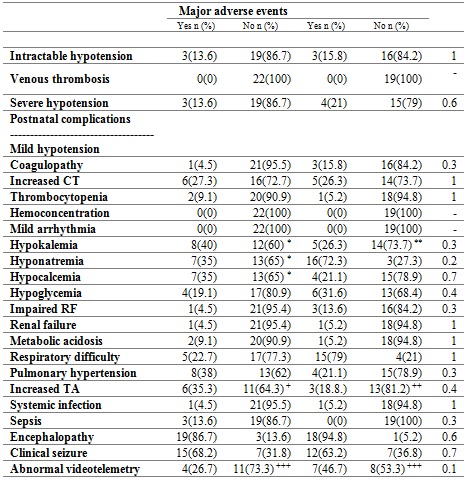
Proportion of patients by type of complication. CT: clotting time. RF: renal function. TA: Transaminases. *Percentage out of a total of 20 patients. **Percentages based on a total of 18 patients. +Percentages based on 17 patients. ++Percentages based on 16 patients. +++Abnormal/Normal. Percentages based on 15 patients. ‡Fisher’s exact test.
The main clinical outcome at discharge was encephalopathy (90%), determined on the basis of three criteria: videotelemetry, brain magnetic resonance taken at 3 and 5 days, respectively, after the end of therapy, and clinical examination performed by pediatric neurology before discharge. Some degree of encephalopathy was considered if there was abnormality in one or more criteria. In our study, follow-up was carried out from admission to therapy until discharge. Of all patients, 63.3% had abnormal videotelemetry, and 51% had abnormal resonance imaging. When comparing complications after the intervention in relation to the average time from birth to initiation of hypothermia, there was only a significant difference in the late initiation of the intervention with the onset of clinical seizures. It is worth mentioning that only 5 patients received the therapy in the first 6 hours (Table 3).
Table 3 Complications of treatment in relation to the time of initiation of care
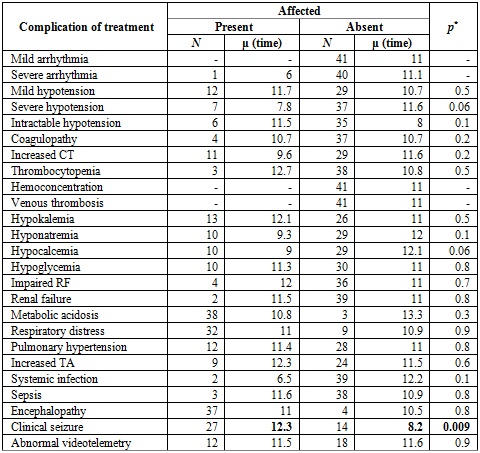
Note: The complications presented after the intervention are compared in relation to the average time in which hypothermia was initiated (average number of hours from birth to initiation of the intervention). The average hours of initiation of care was significantly higher in the case of patients with clinical seizure. CT: clotting time; RF: renal function. TA: Transaminases. *Student's T
Figures 1 and 2 show a comparison of the outcomes of survival or death after the intervention in relation to the same clinical variables of interest for each newborn. Patients with lower Apgar scores at 10 minutes and acidemia had higher mortality, which corresponded to 17%. All patients who died had cord blood gas < 6.91 and base excess > – 19; 14% of the patients who entered the therapy had a pH of 7.0 in umbilical cord blood, and 17% had a PH greater than 7.0.
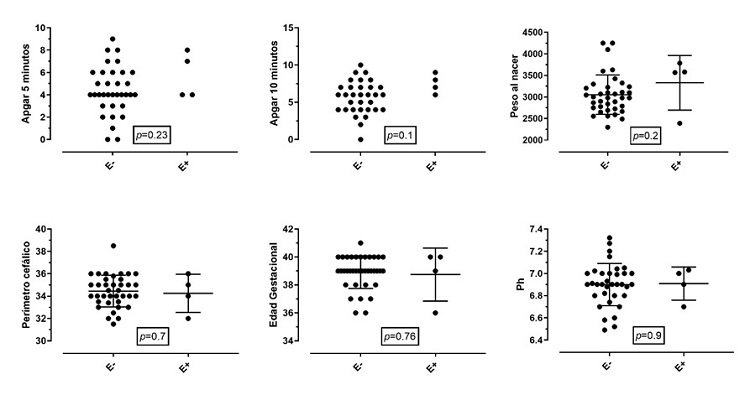
Figure 1 Events of encephalopathy related to clinical variables of interestNote: The clinical determinants of the birth of the newborn are compared with the presence of encephalopathy. Proportionally, no significant differences were found between the two groups. Birth weight in grams. Cephalic perimeter in centimeters. Gestational age in weeks. E+: encephalopathy present. E-: encephalopathy absent. The Apgar graph only shows the events, without mean or SD.
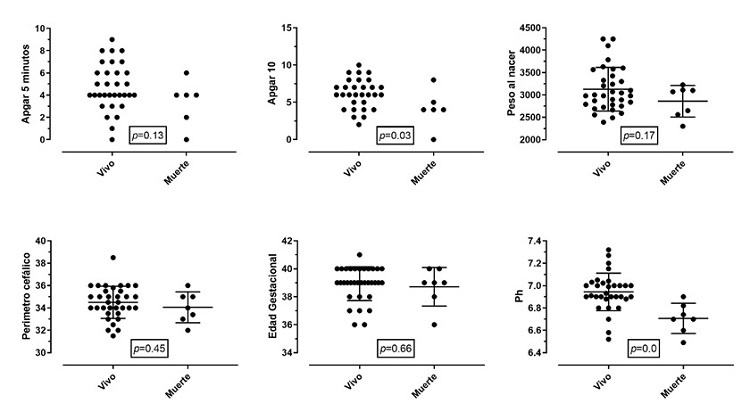
Figure 2 Outcome of patients (alive or dead)Note: The clinical determinants of the birth of the newborn are compared with the outcome of death. Proportionally, no significant differences were found between the two groups, except for 10-minute Apgar and the presence of acidemia. Birth weight in grams. Cephalic perimeter in centimeters. Gestational age in weeks. The Apgar graph only shows the events, without mean or DE.
The outcomes of mortality and encephalopathy were compared in relation to the time of care from the moment of birth until the initiation of the intervention. Figure 3 shows each of the events.
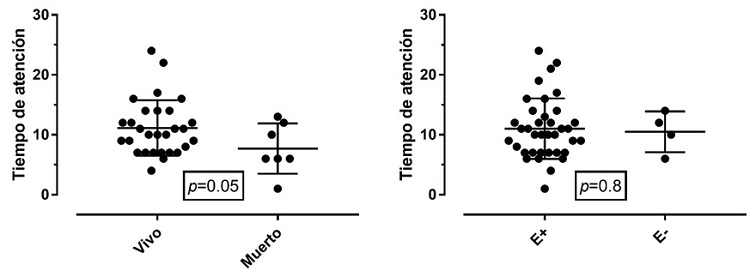
Figure 3 Time of care in relation to mortalityNote: Comparison of the average time for initiation of the intervention after birth in relation to mortality outcomes and the presence of encephalopathy. E+: encephalopathy present. E-: encephalopathy absent.
Table 4 shows the comparison of the prevalence ratio (PR) of encephalopathy and death according to exposures of clinical interest. Regarding the outcome of encephalopathy, PRs > 1 were evidenced in cases of metabolic acidosis, respiratory distress, pulmonary hypertension and clinical seizures. However, 95% CIs go through a PR of 1. For the outcome of death, PRs > 1 are seen in the case of cesarean section, increased clotting time, thrombocytopenia, hypocalcemia and impaired renal function (p < 0.05 and 95% CIs > 1).
Table 4 Prevalence ration between the exposure of variables of clinical interest and the outcomes of encephalopathy and death
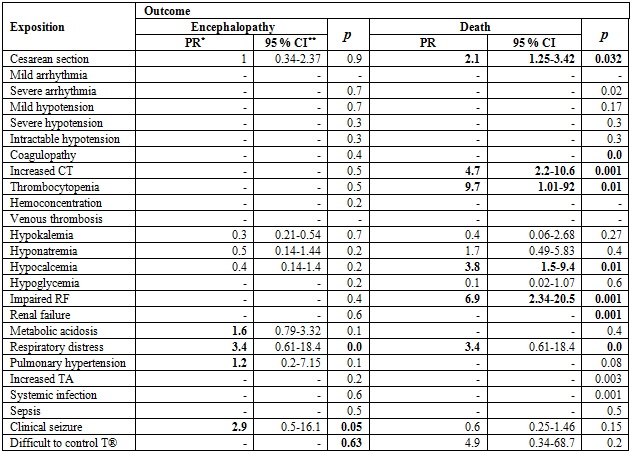
PR: Prevalence ratio. 95% CI: 95% confidence interval. RF: Renal function. TA: transaminases.
One patient had baseline gases compatible with asphyxia, so he entered the protocol, but later it was determined that he had panhipotuitarianism.
Discussion
According to the data provided by the Public Health Surveillance Area of the District Department of Health, based on records from the Unified Registry of Affiliates, the perinatal mortality rate in Bogotá has shown an important decrease, of up to 13.4 per each 1,000 births for 2017. Early neonatal mortality had remained around 5.9 per 1,000 births until 2013 and began to fall to 4.3 per 1,000 births by 2014 (13). These trends are similar to the decrease in mortality in developing countries (14,15), emerging economies (16) and developed countries (17,18). This reduction is due to the control of perinatal infectious pathologies (19) and to the better management of complications derived from childbirth (20) and other newborn pathologies, including congenital malformations (21), as well as to an improvement in resuscitation techniques (22).
Since 2015, the Hospital Universitario San Ignacio is part of the District Neuroprotection Network and offers a proven clinical efficacy management (23) to neonates with moderate or severe asphyxia referred from different health institutions in the District and the department, regardless of their affiliation to social security.
Table 1 shows that the EEG activity before the intervention has significant moderately and severely abnormal results. EEG activity is a sensitive marker of brain damage in both preterm (24) and term infants (25). Encephalopathy is a progressive syndrome, so this tool becomes more and more important and helps us determine if a patient could benefit from entering the protocol reported in the literature with a 75% PPV for an event adverse, and the combination of this with an altered test has a PPV higher than each one separately (26).
Several clinical alterations are produced both by the underlying pathology and by therapy, and cannot be accurately distinguished (27). In our study, however, some of them were predictors of mortality, such as a low 10-minute Apgar, acidemia, history of cesarean section, increased clotting times, thrombocytopenia, hypocalcemia, and impaired kidney function (27).
90% of patients were diagnosed with some degree of encephalopathy at discharge, determined by some alteration in videotelemetry, brain magnetic resonance or neurological physical examination. However, only one of the surviving patients required gastrostomy, and was diagnosed with panhypopituitarism. The others were discharged with adequate sucking. 41.46% of the patients were discharged with mild encephalopathy.
A longer follow-up was not possible, because health insurers do not refer these patients to our institution for control. Unlike the study by Manotas et al. (11) on total body hypothermia, the mortality of our patients was not related to the time of initiation of therapy. All patients who died in our study had a cord blood gas pH < 6.91 and base excess > – 19, and 6 of them had a pH < 6.7. We share thrombocytopenia as a mortality risk factor when it is below 100,000.
Patients who took longer to receive active hypothermia therapy had more seizures and encephalopathy. However, for mortality, the average attention time in both types of outcome was similar. This finding could be considered important to support the entry of patients to hypothermia even after the 6 hours of therapeutic window initially described for the protocol, although with greater risk of having clinical seizures. Recent studies support this hypothesis (28).
14% of the patients who received the therapy had an umbilical cord blood pH equal to 7, and 17%, greater than 7. However, they were admitted to therapy because they met criteria A and B, which leads to the conclusion that it is not the only or most important criterion to define which patients require therapy, but it is considered a predictor of mortality, because none of these patients died. Some patients have differential diagnoses that should be taken into account and that can simulate asphyxia states, as happened with our patient with panhipotuitarianism.
Conclusions
Patients who had a low 10-minute Apgar score and severe acidemia had higher mortality. All patients who died had cord blood gas < 6.91 and excess base > – 19. There was a greater prevalence of clotting, electrolyte and renal alterations in the group with fatal outcome.
The results show that it is a priority to more accurately define the process of timely referral of patients and in the future to have the asphyxia clinic to ensure a comprehensive and multidisciplinary follow-up of patients managed with this therapy. However, in our study we did not find an important difference in mortality when the therapy was started after six hours.
Given the difference in mortality, compared with that of body hypothermia, additional studies are required to determine if in our context the two therapies are equally effective.
Referencias
1.Almeida MFB, Kawakami MD, Moreira LMO, Santos RMVD, Anchieta LM, Guinsburg R. Early neonatal deaths associated with perinatal asphyxia in infants ≥2500g in Brazil. J Pediatr (Rio J). 2017;93(6):576-84. [ Links ]
2.Daripa M, Caldas HM, Flores LP, Waldvogel BC, Guinsburg R, de Almeida MF. Perinatal asphyxia associated with early neonatal mortality: populational study of avoidable deaths. Rev Paul Pediatr. 2013;31(1):37-45. [ Links ]
3.Penela-Vélez de Guevara MT, Gil-López SB, Martín-Puerto MJ, Romero-Escós MD, Herrera-Martín M, Urbón-Artero A. A descriptive study of perinatal asphyxia and its sequelae. Rev Neurol. 2006;43(1):3-6. [ Links ]
4.Secretaría Distrital de Salud. Lineamiento técnico para el manejo de la asfixia perinatal. Bogotá: Secretaría; 2015. [ Links ]
5.Shankaran S. Outcomes of hypoxic-ischemic encephalopathy in neonates treated with hypothermia. Clin Perinatol. 2014;41(1):149-59. [ Links ]
6.Volpe J. Neurología del recién nacido. 5 ed. Madrid: McGraw Hill; 2013. [ Links ]
7.Centro Nacional de Investigación en Evidencia. Guía de práctica clínica del recién nacido con asfixia perinatal. Bogotá; Ministerio de Salud; 2013. [ Links ]
8.Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, Goldsmith JP, et al. Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics. 2010;126(5):e1319-44. [ Links ]
9.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013(1):CD003311. https://doi.org/10.1002/14651858.CD003311.pub3 [ Links ]
10.Shah PS. Hypothermia: A systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med. 2010;15(5):238-46. [ Links ]
11.Manotas H, Troncoso G, Sánchez J, Molina G. Descripción de una cohorte de pacientes neonatos con diagnóstico de asfixia perinatal tratados con hipotermia terapéutica. Perinatol Reprod Hum. 2018;32(2):70-7. [ Links ]
12.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366(22):2085-92. [ Links ]
13.Secretaría Distrital de Salud de Bogotá. Base de datos SDS y aplicativo Web RUAF_ND: datos Preliminares. Bogotá: Secretaría; 2017. [ Links ]
14.Mah-Mungyeh E, Chiabi A, Tchokoteu FL, Nguefack S, Bogne JB, Siyou HH, et al. Neonatal mortality in a referral hospital in Cameroon over a seven year period: Trends, associated factors and causes. Afr Health Sci. 2014;14(4):985-92. [ Links ]
15.Akinyemi JO, Bamgboye EA, Ayeni O. Trends in neonatal mortality in Nigeria and effects of bio-demographic and maternal characteristics. BMC Pediatr. 2015;15:36. [ Links ]
16.Wu QJ, Li LL, Li J, Zhou C, Huang YH. Time trends of neonatal mortality by causes of death in Shenyang. 1997-2014. Oncotarget. 2016;7(13):16610-8. [ Links ]
17.Dallolio L, Lenzi J, Fantini MP. Temporal and geographical trends in infant, neonatal and post-neonatal mortality in Italy between 1991 and 2009. Ital J Pediatr. 2013;39:19. [ Links ]
18.Gregory EC, MacDorman MF, Martin JA. Trends in fetal and perinatal mortality in the United States. 2006-2012. NCHS Data Brief. 2014(169):1-8. [ Links ]
19.Battin MR, Knight DB, Kuschel CA, Howie RN. Improvement in mortality of very low birthweight infants and the changing pattern of neonatal mortality: the 50-year experience of one perinatal centre. J Paediatr Child Health. 2012;48(7):596-9. [ Links ]
20.Shah V, Warre R, Lee SK. Quality improvement initiatives in neonatal intensive care unit networks: Achievements and challenges. Acad Pediatr. 2013;13(6 Suppl):S75-83. [ Links ]
21.Marantz P, Sáenz Tejeira MM, Peña G, Segovia A, Fustiñana C. Fetal and neonatal mortality in patients with isolated congenital heart diseases and heart conditions associated with extracardiac abnormalities. Arch Argent Pediatr. 2013;111(5):418-22. [ Links ]
22.Van Heerden C, Maree C, Janse van Rensburg ES. Strategies to sustain a quality improvement initiative in neonatal resuscitation. Afr J Prim Health Care Fam Med. 2016;8(2):e1-e10. [ Links ]
23.Galvao TF, Silva MT, Marques MC, de Oliveira ND, Pereira MG. Hypothermia for perinatal brain hypoxia-ischemia in different resource settings: a systematic review. J Trop Pediatr. 2013;59(6):453-9. [ Links ]
24.Ranasinghe S, Or G, Wang EY, Ievins A, McLean MA, Niell CM, et al. Reduced cortical activity impairs development and plasticity after neonatal hypoxia ischemia. J Neurosci. 2015;35(34):11946-59. [ Links ]
25.Jose A, Matthai J, Paul S. Correlation of EEG, CT, and MRI brain with neurological outcome at 12 months in term newborns with hypoxic ischemic encephalopathy. J Clin Neonatol. 2013;2(3):125-30. [ Links ]
26.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet. 2005;365(9460):663-70. [ Links ]
27.Wayock CP, Meserole RL, Saria S, Jennings JM, Huisman TA, Northington FJ, et al. Perinatal risk factors for severe injury in neonates treated with whole-body hypothermia for encephalopathy. Am J Obstet Gynecol. 2014;211(1):41 e1-8. [ Links ]
28.Laptook A, Shanakaran S, Tyson J, et al. Effect of therapeutic hypothermia initiated afeter 6 hours of age on death or disability among newborns with hypoxic-isquemic encephalopathy. JAMA. 2017;318(16):1530-60. [ Links ]
Received: November 06, 2018; Accepted: June 05, 2019











 texto em
texto em 


