Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Universitas Medica
versão impressa ISSN 0041-9095versão On-line ISSN 2011-0839
Univ. Med. vol.60 no.4 Bogotá out./dez. 2019
https://doi.org/10.11144/javeriana.umed60-4.oste
Research articles
Characterization of Children with Non-Metastatic Osteosarcoma who Received Treatment with Mifamurtide in Two Institutions in Colombia between 2014 and 2017
1Mécia oncóloga pediatra, Clinica Infantil Colsubsidio-Hospital Militar Central, Bogotá, Colombia.
2Médico hematoncólogo pediatra, Hospital Federico Lleras Acosta, Ibagué, Colombia.
3Médica oncóloga pediatra, Hospital Militar Central, Bogotá, Colombia.
Introducción:
Osteosarcoma is the most frequent bone tumor in children. Survival of patients who do not have metastases at the beginning has not changed in the last decade; there are studies that suggest the benefit of the use of new molecules such as mifamurtide.
Methods:
We described the variables of interest in 8 patients under 18 years of age with high-grade non-metastatic osteosarcoma, who received management with conventional chemotherapy and mifamurtide as adjuvant in 2 Colombian institutions between 2014 and 2017.
Results:
The majority of the patients had femoral compromise due to conventional osteosarcoma, all of them received management with pre and post-surgical chemotherapy, 75% of the patients were taken to limb salvage. In total, 375 cycles of mifamurtide were evaluated (2 mg/m2 of total body surface area). There were adverse effects in 7 of the 375 cycles administered (1.87 %); these occurred in 4 of the 8 patients participating in the study; at the end of the study, 6 of 8 patients were alive.
Conclusions:
In the patients evaluated, the use of mifamurtide was well tolerated, however due to the type of study it cannot be determined if the use of this medication had an impact on survival.
Keywords osteosarcoma; mifamurtide; child; survival
Introducción:
El osteosarcoma es el tumor óseo más frecuente en los niños. La supervivencia de los que no tienen metástasis al inicio del tratamiento no ha cambiado significativamente en la última década. Existen estudios que sugieren el beneficio del uso de nuevas moléculas como mifamurtida.
Métodos:
Se describieron las variables de interés en 8 pacientes menores de 18 años con osteosarcoma de alto grado no metastásico, que recibieron quimioterapia convencional y mifamurtida como adyuvante en 2 instituciones de Colombia entre 2014 y 2017.
Resultados:
La mayoría de los pacientes tenía afectación del fémur por osteosarcoma convencional. Todos se manejaron con quimioterapia pre y posquirúrgica. El 75 % de los pacientes fue llevado a salvamento de extremidad. En total se evaluaron 375 ciclos de mifamurtida a dosis de 2 mg/m2 de superficie corporal total. Se presentaron efectos adversos en 7 de los 375 ciclos administrados (1,87 %), en 4 de los 8 pacientes participantes en el estudio. Al finalizar el estudio, 6 de los 8 pacientes estaban vivos.
Conclusiones:
En los pacientes evaluados, el uso de mifamurtida fue bien tolerado; sin embargo, por el tipo de estudio, no se puede determinar si el uso de este medicamento tuvo impacto en la supervivencia.
Palabras clave osteosarcoma; mifamurtida; niño; supervivencia
Introduction
Bone tumors represent approximately 6% of oncological diseases in people under 20 years of age. Osteosarcoma is the most frequent malignant bone tumor in children and adolescents, and corresponds to 56% of all bone neoplasms in this population. The annual incidence ranges from 3.5 cases per million in children under 15 years of age to 8.8 cases per million in people between 15 and 19 years of age, with a peak of appearance around 15 years of age (1,2). In Colombia, the Cali population register reported an incidence adjusted by age and sex of 4.2 cases per million in children under 15 years of age (3).
At the time of diagnosis, 70%-80% of patients had localized disease, and 20-30% had metastases, the most frequent of which were lung, lymph and bone metastases (4).
Current treatment of metastatic and non-metastatic high-grade osteosarcoma includes neoadjuvant chemotherapy, followed by complete surgical resection of the primary tumor, ideally with negative margins, and adjuvant chemotherapy. The choice of the chemotherapy regimen and the optimal moment (i.e., preoperative-neoadjuvant versus postoperative-adjuvant) has been controversial, since there is no definitive survival benefit for neoadjuvant chemotherapy compared to adjuvant chemotherapy (5). However, many centers prefer to use preoperative chemotherapy, particularly if a limb preservation procedure is being considered.
The degree of necrosis in the pathological anatomy reflects the effectiveness of the neoadjuvant chemotherapy (4). The response to neoadjuvant chemotherapy is an important prognostic factor, but there is no evidence that better results are achieved in patients with poor histological response to neoadjuvant chemotherapy by altering the postoperative chemotherapy regimen (6). The degree to which an osteosarcoma responds to neoadjuvant chemotherapy is an important determinant of clinical outcome for most histological subtypes (7,8,9,10,11,12,13,14,15).
Five-year survival rates for patients with an extremity sarcoma and a “good” response to chemotherapy (as defined by 95% or more of necrosis in the surgical specimen) are significantly higher than those with a lesser response (71%-80% vs. 45%-60%, respectively) (11,13,15,16). However, the demonstration of an inverse relationship between the predictive value of tumor necrosis and the intensity of induction therapy in a report has led to question the true value of the histological response as a prognostic marker (17).
The therapeutic objective is to eliminate clinically detectable tumors and to control microscopic metastases with polychemotherapy, to improve survival and prevent recurrence. It was assumed and subsequently demonstrated that subclinical metastatic disease is present at the time of diagnosis in most patients, and that chemotherapy can successfully eradicate these deposits if it is started when the disease load is low (18).
The most commonly used drugs are: cisplatin, doxorubicin, methotrexate with leucovorin, ifosfamide and etoposide rescues (19,20). The optimal regime has not been established. However, the available evidence supports the benefit of a three-drug regimen compared to a two-drug regimen, particularly for children and young adults (21).
For children and adolescents, we recommend the methotrexate, doxorubicin and cisplatin (MAP) regimen, which was used in the control arm of the American Osteosarcoma Study Group (AOST) 0331 (EURAMOS-1) protocol (22).
The prognosis, mortality and progression rate are mainly related to the presence of metastasis (23). The 5-year event-free survival has not changed significantly in the last 20 years (24). With the current treatment, it ranges from 60%-70% for patients with non-metastatic osteosarcoma, and from 20%-30% for those with metastatic osteosarcoma (4). Therefore, new therapeutic strategies are required to improve these patients’ survival. In this scenario, a modification of the postoperative regimen was considered in patients who did not achieve adequate necrosis rates, and the pioneer was the T10 protocol of the Memorial Sloan-Kettering Cancer Center (25,26).
However, this consideration stopped being used, because the benefits of the T10 strategy were not maintained (27). The main cooperative groups have not been able to confirm that these changes improve the results (17,28,29,30,31), and the multi-group international trial EURAMOS-1 showed no benefit of changing the schemes in patients with poor histological responses (6).
In this context, other considerations have been to study immunomodulatory drugs with antitumor action, especially in conventional chemotherapy-resistant neoplasms (19,24). Mifamurtide (muramyl tripeptide: MPT) is a lipophilic synthetic analog of muramyl dipeptide, a component of the bacteria cell walls, with immunostimulating properties. This drug increases the production of proinflammatory cytokines. As it is lipophilic, it is incorporated into the membrane of macrophages and monocytes, which are specifically activated against tumor cells, without affecting normal cells. MTP helps to eradicate residual micrometastases that exist in patients with chemotherapy-resistant osteosarcoma (4,24,32).
The recommended dose of MTP is 2 mg/m2 of body surface area, administered intravenously in 1 hour, 2 times a week for 12 weeks; then, once a week for 24 weeks, for a total of 36 weeks and 48 doses (4).
There is interest in continuing to study the efficacy of this agent as standard therapy in osteosarcoma (33,34,35). Some studies suggest that the addition of MTP improves event-free survival in patients with non-metastatic osteosarcoma (23,33). There are no studies in upper-middle, lower-middle or low income countries with this drug in children with osteosarcoma.
In relation to adverse effects and possible toxicity, hematological, hepatic, renal, digestive, cardiac, and nervous system effects have been reported, as well as hearing loss, fever and infection (4).
MTP has been available in Colombia since 2014. In 2016 the Clínica Infantil Colsubsidio and the Hospital Militar Central, in Bogotá, included it in the management protocol for children with non-metastatic osteosarcoma. In this article, we characterize patients with non-metastatic osteosarcoma who received MTP as part of the oncological treatment in these two institutions.
Materials and methods
We present a case series (observational, descriptive and retrospective study) with all patients aged 2 to 18 years with high-grade, non-metastatic osteosarcoma who underwent macroscopically complete surgical resection and started treatment with MTP in addition to the conventional protocol, from January 1, 2014 to July 31, 2017, in two institutions that handle cancer patients in Bogotá (Colombia).
Parents of the included patients gave their informed consent, and also the patients, in the pertinent cases. After obtaining the authorization from the Ethics and Research Committee of the participating institutions, we reviewed the clinical histories with the help of the principal investigators and the register of the value of the variables with an Excel® database.
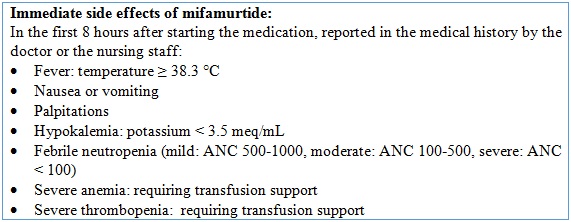
We evaluated the patients’ general and sociodemographic characteristics, important oncological aspects, tumor histology and location, and the oncological treatment provided (which included cumulative dose of chemotherapy and local control type). We also collected information about the side effects mentioned in Table 1.
The information was collected and analyzed using Excel®. For the qualitative variables, frequency measures were estimated, and for the quantitative variables measures of central tendency were calculated: average or median with the corresponding dispersion measures.
Results
Eight patients were evaluated, corresponding to all children diagnosed with non-metastatic osteosarcoma who were admitted to the participating institutions. The results are presented in tables 2 and 3.
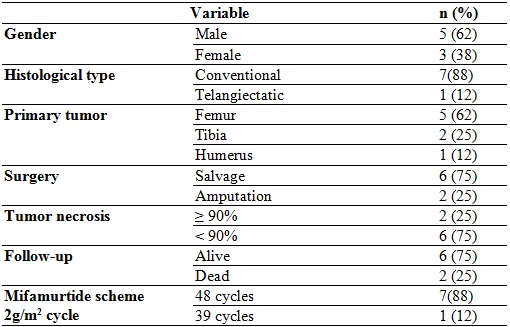
The average age of the patients at the time of diagnosis was 12.5 years (with an interval of 9 to 16 years), the majority were men and the most frequent location of the primary tumor was the distal femur. The most common histological type was central osteosarcoma, conventional subtype (according to the World Health Organization classification).
All of them received neoadjuvant chemotherapy prior to local control. A good response was considered when the percentage of necrosis reached was greater than 95%, according to the Huvos classification. Subsequently, adjuvant chemotherapy was administered.
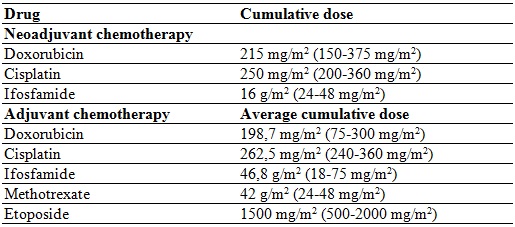
The neoadjuvant chemotherapy regimen provided was doxorubicin and cisplatin. Six patients were administered ifosfamide and methotrexate, depending on the clinical stage, the patient’s adherence and the institutional protocol. Only 2 patients required amputation; the others underwent salvage surgery. All received adjuvant chemotherapy with doxorubicin; 7 received ifosfamide and cisplatin; 6 methotrexate, and 5 etoposide. All received MTP 2 mg/m2 per cycle, 7 completed 48 cycles, and one received 39 cycles.
At the end of the follow-up, in August 2018, 2 patients had died due to disease progression with pulmonary metastatic involvement; both had less than 95% necrosis at the time of local control, and one of them was the one that received 39 cycles of mifamurtide.
There were adverse effects in 7 of the 375 cycles administered (1.87%); these occurred in 4 of the 8 patients participating in the study. Three patients presented with fever, anemia, thrombocytopenia and neutropenia in the first 6 hours of application in one of the cycles. One patient presented with fever not associated with alterations in the blood count; one patient presented with fever associated with mild anemia, and one presented headache. Finally, one patient reported palpitations.
Discussion and conclusions
Although the incidence of osteosarcoma is low, it is the most frequent malignant bone tumor in children. Many cases are diagnosed late, which greatly influences the prognosis.
The treatment plan continues to be local surgical control with chemotherapy before and after the procedure, with conventional medications such as alkylating agents, platinum, anthracyclines, semisynthetic podophyllin and folic acid analogues.
Despite the scientific advances and the adjustments made to the treatment protocols, survival has not changed significantly in recent decades, which makes it necessary to consider therapeutic strategies with different mechanisms of action that influence the prognosis of the kids. MTP has immunomodulatory properties that confer it tumoricidal activity and there are studies that suggest its effectiveness in patients with osteosarcoma.
Meyers et al. (33) published a clinical trial in 2008 to determine if the addition of ifosfamide and MTP increased overall survival and event-free survival in patients with non-metastatic, resectable osteosarcoma. They made a 2 × 2 factorial design, including 662 patients. They compared chemotherapy with cisplatin, doxorubicin and methotrexate with and without the addition of ifosfamide and MTP. The addition of MTP improved the 6-year survival from 70% to 78% (p = 0.03). The risk ratio was 0.71 (95% CI: 0.52-0.96). They did not find statistically significant differences between the scheme with ifosfamide and without it. They concluded that the addition of MTP improved overall survival.
In 2017, Jimmy et al. (4) published a systematic review of the literature to estimate the effect of administering MTP in addition to adjuvant chemotherapy, on event-free survival, overall survival, recurrence and quality of life in patients with metastatic and not metastatic high-grade osteosarcoma. They found two experimental studies that included 802 patients of all ages. No differences were found in event-free survival in metastatic and non-metastatic osteosarcoma. There were small, but statistically significant differences in favor of MTP in overall survival in patients with non-metastatic osteosarcoma and in progression-free survival in patients with pulmonary metastases or relapse. The authors concluded that there is little evidence to estimate the effectiveness of MTP.
The 2015 study by Song et al. (23) used a Markov model to estimate the effectiveness of adding MTP to chemotherapy in patients with metastatic and non-metastatic osteosarcoma, with a lifelong time horizon, starting at 13 years of age. The study concluded that the addition of MTP increases the treatment’s effectiveness in metastatic and non-metastatic osteosarcoma. The relative effectiveness was higher in metastatic osteosarcoma.
Published studies include populations of different ages, without considering children as a subgroup. There are no studies on the effectiveness and side effects of MTP in upper-middle or low-income countries. In our study, 375 cycles were administered to 8 patients with non-metastatic osteosarcoma. Two of these patients died, and side effects occurred in only 1.87% of the cycles. There were no deaths associated with drug toxicity. It is important to mention that we took into account patient, physician and nursing staff reported events, without making an active search for other events documented in the literature on the subject.
Considering that it is a descriptive study, its results are aimed at generating hypotheses. However, in view of the low prevalence of the disease and the fact that there are no studies in developing countries, the information about the experience with children treated with MTP in the mentioned institutions is valuable for the scientific community. Although in Colombia other institutions besides the ones mentioned in the present study have used MTP, no study has been published in this regard.
Referencias
1. Lanzkowsky P, Lipton JM, Fish JD. Manual of pediatric hematology and oncology. 6th ed. s. l.: Academic Press; 2016. [ Links ]
2. Pizzo PA, Poplack DG. Principles and practice of pediatric oncology. 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2015. [ Links ]
3. Suárez A, Soto C, Gómez L, Gamboa Ó, Soto D, Escandón S, et al. Resultados del tratamiento de osteosarcoma convencional de alto grado en niños y adolescentes: análisis de supervivencia de una cohorte tratada sin metotrexato. Rev Colomb Cancerol. 2017;21(2):86-94. [ Links ]
4. Jimmy R, Stern C, Lisy K, White S. Effectiveness of mifamurtide in addition to standard chemotherapy for high-grade osteosarcoma. JBI Database Syst Rev Implement Reports. 2017 Aug;15(8):2113-52. [ Links ]
5. Goorin AM, Schwartzentruber DJ, Devidas M, Gebhardt MC, Ayala AG, Harris MB, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21(8):1574-80. [ Links ]
6. Marina NM, Smeland S, Bielack SS, Bernstein M, Jovic G, Krailo MD, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): An open-label, international, randomised controlled trial. Lancet Oncol. 2016 Oct;17(10):1396-408. [ Links ]
7. Rosen G. Preoperative (neoadjuvant) chemotherapy for osteogenic sarcoma: A ten year experience. Orthopedics. 1985;8(5):659-64. [ Links ]
8. Bielack SS, Kempf-Bielack B, Winkler K. Osteosarcoma: Relationship of response to preoperative chemotherapy and type of surgery to local recurrence. J Clin Oncol. 1996;14(2):683-4. [ Links ]
9. Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12(12):2699-705. [ Links ]
10. Kawai A, Healey JH, Boland PJ, Lin PP, Huvos AG, Meyers PA. Prognostic factors for patients with sarcomas of the pelvic bones. Cancer. 1998;82(5):851-9. [ Links ]
11. Bacci G, Bertoni F, Longhi A, Ferrari S, Forni C, Biagini R, et al. Neoadjuvant chemotherapy for high-grade central osteosarcoma of the extremity. Cancer. 2003;97(12):3068-75. [ Links ]
12. Bacci G, Picci P, Ruggieri P, Mercuri M, Avella M, Capanna R, et al. Primary chemotherapy and delayed surgery (neoadjuvant chemotherapy) for osteosarcoma of the extremities. The Istituto Rizzoli Experience in 127 patients treated preoperatively with intravenous methotrexate (high versus moderate doses) and intraarterial cisplatin. Cancer. 1990;65(11):2539-53. [ Links ]
13. Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776-90. [ Links ]
14. Petrilli AS, de Camargo B, Filho VO, Bruniera P, Brunetto AL, Jesus-Garcia R, et al. Results of the Brazilian Osteosarcoma Treatment Group Studies III and IV: Prognostic factors and impact on survival. J Clin Oncol. 2006;24(7):1161-8. [ Links ]
15. Hauben EI, Weeden S, Pringle J, Van Marck EA, Hogendoorn PC. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? Eur J Cancer. 2002;38(9):1218-25. [ Links ]
16. Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy. Cancer. 2006;106(5):1154-61. [ Links ]
17. Bishop MW, Chang Y-C, Krailo MD, Meyers PA, Provisor AJ, Schwartz CL, et al. Assessing the prognostic significance of histologic response in osteosarcoma: A comparison of outcomes on CCG-782 and INT0133-A report from the Children’s Oncology Group Bone Tumor Committee. Pediatr Blood Cancer. 2016;63(10):1737-43. [ Links ]
18. Bruland OS, Høifødt H, Saeter G, Smeland S, Fodstad O. Hematogenous micrometastases in osteosarcoma patients. Clin Cancer Res. 2005;11(13):4666-73. [ Links ]
19. Frampton JE. Mifamurtide: A review of its use in the treatment of osteosarcoma. Pediatr Drugs. 2010 Jun;12(3):141-53. [ Links ]
20. Meyers PA. Muramyl tripeptide (mifamurtide) for the treatment of osteosarcoma. Expert Rev Anticancer Ther. 2009 Aug 10;9(8):1035-49. [ Links ]
21. Anninga JK, Gelderblom H, Fiocco M, Kroep JR, Taminiau AHM, Hogendoorn PCW, et al. Chemotherapeutic adjuvant treatment for osteosarcoma: Where do we stand? Eur J Cancer. 2011;47(16):2431-45. [ Links ]
22. Clinical Trials. Combination chemotherapy, PEG-interferon alfa 2b, and surgery in treating patients with osteosarcoma [internet]. 2015 [Citado 2011 jul 19]. Disponible en: http://www.clinicaltrials.gov/ct2/show/NCT00134030?term=AOST+0331&rank=1 [ Links ]
23. Song HJ, Lee JA, Han E, Lee E-K. Lifetime effectiveness of mifamurtide addition to chemotherapy in nonmetastatic and metastatic osteosarcoma: a Markov process model analysis. Tumor Biol. 2015 Sep 3;36(9):6773-9. [ Links ]
24. Ando K, Mori K, Corradini N, Redini F, Heymann D. Mifamurtide for the treatment of nonmetastatic osteosarcoma. Expert Opin Pharmacother. 2011 Feb 13;12(2):285-92. [ Links ]
25. Rosen G, Marcove RC, Huvos AG, Caparros BI, Lane JM, Nirenberg A, et al. Primary osteogenic sarcoma: Eight-year experience with adjuvant chemotherapy. J Cancer Res Clin Oncol. 1983;106(S1):55-67. [ Links ]
26. Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49(6):1221-30. [ Links ]
27. Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcove R, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10(1):5-15. [ Links ]
28. Saeter G, Alvegård TA, Elomaa I, Stenwig AE, Holmström T, Solheim OP. Treatment of osteosarcoma of the extremities with the T-10 protocol, with emphasis on the effects of preoperative chemotherapy with single-agent high-dose methotrexate: a Scandinavian Sarcoma Group study. J Clin Oncol. 1991;9(10):1766-75. [ Links ]
29. Winkler K, Beron G, Delling G, Heise U, Kabisch H, Purfürst C, et al. Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol. 1988;6(2):329-37. [ Links ]
30. Souhami RL, Craft AW, Van der Eijken JW, Nooij M, Spooner D, Bramwell VH, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: A study of the European Osteosarcoma Intergroup. Lancet. 1997;350(9082):911-7. [ Links ]
31. Smeland S, Müller C, Alvegard TA, Wiklund T, Wiebe T, Björk O, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: Prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39(4):488-94. [ Links ]
32. Anderson PM, Tomaras M, McConnell K. Mifamurtide in osteosarcoma - A practical review. Drugs of Today. 2010 May;46(5):327. [ Links ]
33. Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J Clin Oncol. 2008 Feb 1;26(4):633-8. [ Links ]
34. Bielack SS, Marina N, Ferrari S, Helman LJ, Smeland S, Whelan JS, et al. Osteosarcoma: The Same Old Drugs or More? J Clin Oncol. 2008;26(18):3102-3. [ Links ]
35. Hunsberger S, Freidlin B, Smith MA. Complexities in interpretation of osteosarcoma clinical trial results. J Clin Oncol. 2008;26(18):3103-4. [ Links ]
Received: March 13, 2019; Accepted: April 22, 2019











 texto em
texto em