Introduction
Intracranial aneurysms (IA) are a major cause of disease and disability due to aneurysm rupture. Although the clinical setting of subarachnoid hemorrhage (SAH) may have been identified by Hippocrates (1), the first to fully describe this event as an entity was Dr. Charles Symonds, who coined the term spontaneous subarachnoid hemorrhage (2). After Dr. Dandy reported the first successful surgical treatment of an IA in 1932 (3), open surgery became the standard of care. However, the high complication and neurological morbidity rates associated with the procedure (including paresis, language dysfunction, long-term and cognitive decline, etc.), led to the development of less invasive techniques. Several endovascular techniques have been described since the late 1960s, using diverse endovascular techniques including balloon angioplasty (4), short coils (5), and electro thrombosis (6). Despite significant advances in endovascular technology and treatment modalities, the treatment paradigm did not begin to shift until 1991, when the first patient was treated using platinum-based detachable coils (7,8). However promising the results were, many cerebrovascular surgeons remained skeptical regarding the true long-term benefits of endovascular techniques. Some saw it as a novel treatment with no long-term efficacy; others simply preferred the “traditional” treatment technique (9). It was this skepticism that led to the publication of several studies comparing these treatment alternatives in terms of safety and long-term outcomes (10,11,12,13). In 2004, many believed the controversy to have ended when the ISAT study showed endovascular coiling to be superior to surgical clipping (14). Endovascular treatment rates skyrocketed, and public interest grew around this minimally invasive approach. However, subsequent studies have shown mixed results (15), and several renowned surgeons questioned the conclusions of the ISAT study (16,17). Hence, the controversy is far from over and research efforts are still ongoing in an attempt to identify the superior treatment option (if any) (18,19,20). Several studies have approached this dichotomy from an economic standpoint. Some have evaluated the hospital costs associated with each treatment (10); others have evaluated the costs associated with the treatment itself in terms of the quality of life (21). Nevertheless, these studies have focused on short-term outcomes, which induce an analytical bias as long-term disability costs are not considered. In the current study, a long-term cost-effectiveness analysis was performed in an attempt to elucidate the health economics associated with these treatment alternatives. The results of the study can assist surgeons and patients by providing an additional tool in the treatment decision process and will also aid the healthcare system in ensuring that limited resources are employed in the most effective manner.
Methods
A decision-tree model was built based on a hypothetical cohort of 1,000 50-year-old theoretical patients with no prior medical history except controlled systemic hypertension who present with an asymptomatic, unruptured anterior circulation aneurysm that warrants treatment (19,22,23,24). Intervention indications were deemed to be beyond the scope of this study and were thus neglected. The model was based on the premise that both surgical clipping and endovascular coiling are equally viable. Health outcomes and cost analysis were modeled over the projected lifetime of the hypothetical cohort. The final measure of effectiveness was defined as achieving complete aneurismal occlusion without procedure-related morbidity or mortality. A decision-tree model was preferred over alternate cost-effectiveness modeling alternatives, such as a Markov model, since patient probabilities were unidirectional and no decision overlap was possible (25).
Patient information, including male-to-female ratio, age, aneurysm size and location, treatment outcome probabilities, follow-up, and likelihood ratios were obtained from the International Study on Unruptured Intracranial Aneurysms (ISUIA) (26). The likelihood of disability and its severity were obtained from ruptured aneurysm trials, as these were the only available sources (14). Information was obtained in a theoretical manner from these studies; no actual patient intervention or results were obtained. Since disability results could vary between ruptured and unruptured lesions, the figures obtained were subjected to a sensitivity analysis and presented before experts with their consensus used in the final model. Unfavorable disability outcomes were defined as a modified Rankin Scale (mRS) score of 3 or higher (27,28). Since the long-term cost of a patient with a mRS score of 3 is different from that of a patient with complete dependence (mRS=5), specific disability probabilities were obtained as described above. Cost-generating events associated with rehabilitation and disability were obtained from the stroke guidelines by the American Heart Association (29) and from the stroke rehabilitation guidelines by the National Institute for Healthcare Excellence (UK) (30). If a specific event was evaluated in both guidelines with different recommendations, the costliest option was used in the model; however, both figures were obtained for the sensitivity analysis. Figure 1 shows the final construct of the decision-tree, along with the different outcome probabilities for each possible scenario.
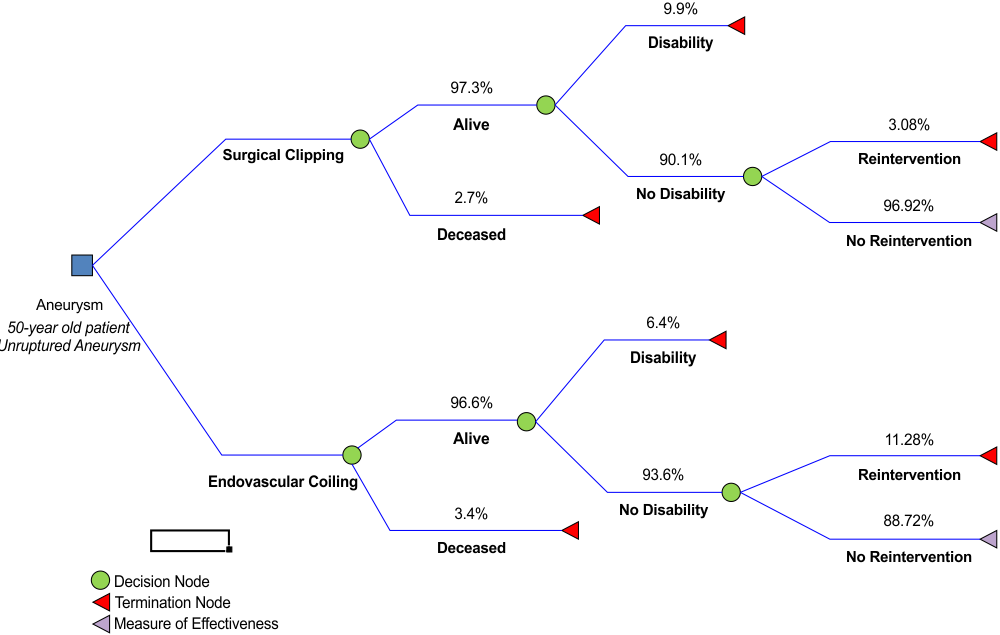
Figure 1 Decision tree model showing the different outcome probabilities according to each treatment option is depicted. Each probability is shown as a percentage above the outcome scenario
Costs were assessed from a societal perspective and were obtained from the costs incurred in the general healthcare system in Colombia. All the costs related to each treatment were obtained from hospital bills generated at our institution in 2015. Disability and rehabilitation costs were obtained from publicly available, inflation-adjusted manuals from 2001. Indirect costs (i.e., the cost of a patient not being able to work) were not evaluated. Costs were calculated in Colombian Pesos and converted into U.S. Dollars using the 2015 average exchange rate (1 USD = 3155 COP).
After the model was run and results were obtained, a univariate sensitivity analysis was conducted in order to analyze the impact of different cost-related and probability variables on the final result. The model was run iteratively, changing the value of each cost and outcome variable (surgical morbidity, surgical mortality, supply costs, operating room costs, etc.) independently between a minimum and maximum value obtained from the literature (31,32). Based on the results of the univariate analysis, a multivariate analysis was deemed unnecessary since the effects of each variable during the univariate analysis did not alter the cost-effectiveness relation. Even when variables were taken to extreme opposites (e.g., the highest reported surgical mortality with the lowest endovascular mortality) the significant difference in the results remained. Therefore, the statistical probability of a multiplicative effect among the variables with a significant impact on the results was negligible.
Results
Cost-effectiveness. Significant differences were found in both treatments cost and total effectiveness. A total treatment effectiveness rate was obtained for both treatment branches. This rate reflects the percentage of patients that do not present with any type of complication or require further intervention. A total treatment effectiveness rate of 84.9% was obtained for surgical clipping, while endovascular coiling had a lower, but still impressive rate of 80.2% (p=0.0056). The cost analysis showed significant differences in both mean treatments cost and the cost-effectiveness ratio. Mean treatment cost refers to the amount paid for the treatment itself (including all supplies as well as surgeon and anesthesia fees) and the hospital stay. The cost-effectiveness ratio is the total cost of each patient that has a successful and effective treatment. Although theoretical, the ratio shows a perceptible relation between the cost of an intervention and its effectiveness, allowing for a quantitative difference to be shown. Surgical clipping was shown to have a mean treatment cost of US$2,322 and a cost-effectiveness ratio of US$2,735, while for endovascular coiling the figures were US$4,650 and US$5,798, respectively (p=0.0078 and 0.065, respectively). These differences were both statistically and economically significant. Due to surgical clipping being both more effective and less costly, no incremental cost-effectiveness ratio was obtainable that granted the alternative treatment a dominant effect. The final cost-effectiveness results are shown in Table 1.
Table 1 Treatment cost per patient, total treatment effectiveness rate, cost difference (compared to surgical clipping), cost and effectiveness differences (compared to surgical clipping) and total cost-effectiveness (the total cost entailed to be able to treat a patient without complications or retreatment)
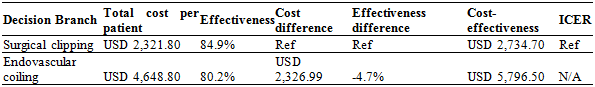
Note.Incremental cost-effectiveness ratio (ICER) is non-applicable since one option is both cheaper and more effective.
Ref: refe rence value.
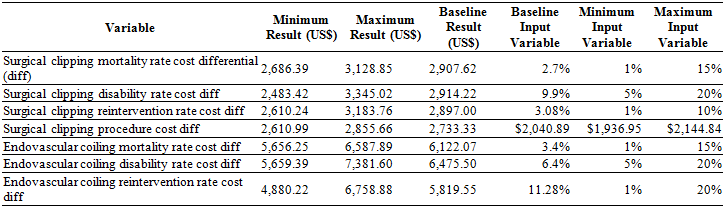
Sensitivity analysis. As clipping was shown to be the dominant treatment alternative, the sensitivity analysis was performed using only the variables of this branch. Table 2 shows the variables used towards the sensitivity analysis and the range assigned to each individual variable along with the minimum and maximum cost-effectiveness result obtained when altering the value of each variable within this range. As mentioned in the methods, a multivariate sensitivity analysis was deemed unnecessary since the effects of varying each variable during the univariate analysis did not alter the results
Discussion
A dominant cost-effectiveness treatment alternative is one that is superior in all evaluated economical aspects. It is both cheaper and more effective than other treatment methods, and therefore is said to dominate the results obtained. According to our findings, surgical clipping is a dominant cost-effective treatment for unruptured IAs in the context of a middle-income country´s healthcare system.
Successful treatment of unruptured IAs is of paramount importance. Patients are usually asymptomatic and the treatment objective is to prevent a potential, not-yet occurred complication. Patients are often skeptical about the need to treat something they do not feel and consequently, the decision-making process for treating an aneurysm is a very complex task. Oftentimes, it is difficult to account for important factors. For instance, a given patient may appear to be an ideal surgical candidate, but refuses to undergo surgery, or a family history of SAH may not be known or available (33). Hence, this study is simply an additional tool in the convoluted endeavor one faces when proposing the best treatment option to each individual patient. However, from a purely economic standpoint, the current results make an enticing argument favoring surgical clipping.
Two variables had a high, albeit economically insignificant, impact on our results. The surgical morbidity (disability) rate and the inherent costs of endovascular coiling (in particular, the cost of the endovascular supplies) were far more influential upon the final results than any other variable evaluated. Regardless of how much these variables were altered (within the realm of possibility), the final cost-effectiveness ratio was constant, rendering these variables economically insignificant.
The current results are consistent with a study by Hoh et al. (34) in which patients with both ruptured and unruptured aneurysms were followed and their hospital cost, length of stay, and reimbursement were analyzed. As in the current study, endovascular coiling was found to result in higher costs, mainly due to the higher device cost of the coils. Nevertheless, as other studies have noted, when a broader analysis is made, endovascular coiling may have an advantage. In a study by Takao et al. (24), an analysis of quality-adjusted life-years showed an economic superiority of endovascular treatment for specific aneurysm locations and sizes (7-24 mm anterior circulation aneurysms and posterior circulation lesions of less than 7 mm). Other researchers have focused their analysis on cumulative costs. In a major long-term follow-up study, Gonda and coworkers (35) showed that an endovascular cost advantage when analyzing cumulative hospital costs was maintained even though patients often required subsequent hospitalizations for reintervention. However, only hospital-related costs were analyzed, excluding important factors such as the cost associated with rehabilitation services and supplies that impaired patients require.
One drawback of the current study concerns the fact that costs were sourced from a single-country healthcare system. Although the evaluation of costs in every single healthcare system worldwide is nearly impossible, we believe this model should be replicated using data from other healthcare systems. Another major drawback is the fact that the evidence used to construct our decision-tree was not of the same origin as the economic data. Alas, there aren’t any major outcome and complication studies in comparable middle-income economies available in the literature. Therefore, we were unable to construct our model with information other than that published in the literature. Nevertheless, we are prospectively collecting a multi-center database on aneurysm outcomes and shall eventually publish a similar cost-effectiveness analysis based on these findings.
Finally, cost and cost-effectiveness comparisons are of interest, not only from an academic standpoint, but also from an international public-health point of view. Endovascular supply costs, for example, vary greatly between countries, directly impacting economic feasibility and potentially making one treatment more economically enticing in a specific macroeconomic setting. Further analysis and model replications will be performed once cost information is available for different countries of interest. Another limitation that warrants mention concerns the fact that the analysis was conducted from a purely economic standpoint. However, as mentioned previously, economic factors are one of many aspects that cerebrovascular surgeons must consider in the multifaceted endeavor involved in treating an unruptured IA.
Conclusions
From an economic standpoint and in consideration of long-term outcomes, surgical clipping is the dominant cost-effective treatment alternative in a middle-income country. The results of the present study can serve as an additional tool in the decision-making process for the treatment of IA, especially in middle-income countries where cost-effective measures are of vital importance. Nevertheless, many other variables must also be taken into account during the decision-making process. Regardless of how important the clinical variables are, the economic implications of our decisions must be considered. Finally, further studies are warranted in various healthcare-system settings of differing income and development tiers because results may vary in different settings.