Introduction
Envenomation by venomous animals is a public health problem, and the morbidity and mortality associated with cases of envenomation by different animals has an impact on the population and the Colombian health system.
The first records of accidents due to contact with caterpillar spines are found in Brazil in 1912 and were described by Zoroastro. These accidents occur mainly in rural areas, and most often affect the upper extremities of patients (1,2). The increase in accidental envenomation with caterpillar venom is associated with deforestation and the secondary elimination of natural predators (2,3).
The main symptom of Lonomia envenomation is an alteration in the clotting times associated with a consumption coagulopathy that leads to hemorrhagic symptoms (1-4). Additionally, it has been described that the venom causes nephrotoxicity and a broad clinical spectrum of deterioration of renal function in both human beings and in vitro studies (3). Although secondary coagulopathy is what occurs most frequently, acute kidney injury is one of the leading causes of death by envenomation. In 1980, 18% of patients developed acute kidney injury, and 50% of them died, but there was an incidence of less than 5.2% (3).
Despite the low incidence of cases of acute kidney failure, it is necessary to understand the pathophysiological mechanism, diagnosis and treatment of this complication to expand awareness and reduce acute kidney failure as a progression of envenomation, and death.
Case Report
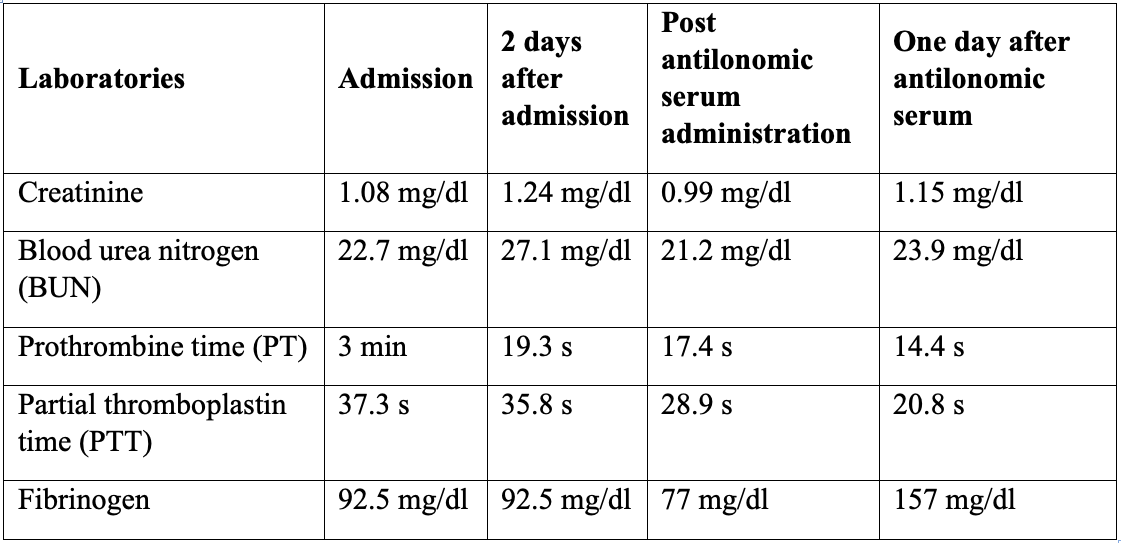
An 80-year-old male patient from Puerto Gaitán (Meta-Colombia), with no relevant medical history, who presents a clinical course of 4 days of evolution, consisting of pain in the left flank and macroscopic hematuria subsequent to contact with poisonous caterpillar (Figure 1). On admission, the patient is hemodynamically stable, with pain on palpation of the left flank, without signs of peritoneal irritation without any other positive finding. The evolution of the laboratories is seen in Table 1. After clinical and paraclinical following and improvement of the patients, discharge occurs.
Methodology
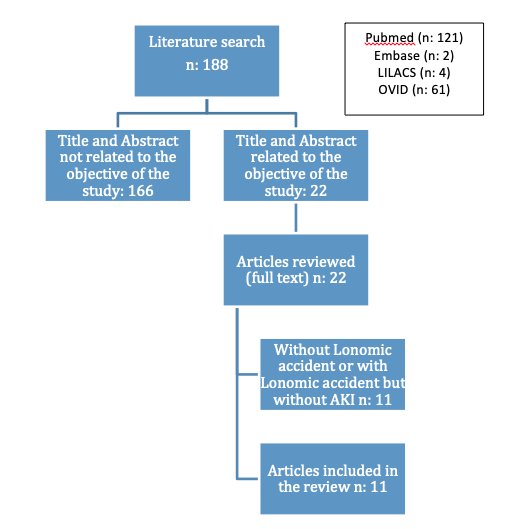
We carried out a scoping review in the Pubmed, Embase, OVID, and LILACS databases. The search strategy was performed using the terms: “Acute Kidney Injury” [Mesh]) AND “lonofibrase” [Supplementary Concept]) OR lonomia accident) OR lonomia. Articles published from January 1, 1998 to November 25, 2018 were include. We included case reports, case series, cohort studies, cases and controls, experimental and cross-sectional studies in English, Spanish, Portuguese, French and Italian that included patients older than 18 years with acute kidney injury, defined as an increase in serum creatinine ≥ 0.3 mg/dL in 48 hours or an increase in serum creatinine ≥ 1.5 times the baseline value or a urine output < 0.5 ml/kg/h for 6 hours (5) secondary to Lonomia envenomation. We excluded articles in which creatinine or urine output values were not documented. The search was conducted separately by two researchers, DMA and MJO, using the search strategy described above. 1 describes the articles included and excluded by title, abstract and finally in the complete revision of the same.
Results
We identified ten patients reported to have be envenomed by L. spp. and one cohort study that included 37 patients who were exposed to the L. obliqua venom. All of them were ranged between 26 and 70 years of age. All the cases were reported to have happened in rural areas. In nine patients, L. obliqua was identified as the cause of envenomation, and only in one case it was due to L. achelous. The common clinical features of these patients at the arrival of the emergency department were the acute kidney injury and the alteration in the clotting times with secondary hemorrhagic manifestations of different localizations. Only one patient was pregnant at the time of exposure to the venom who performed with placental abruption. The summary of the included studies is seen in Table 2.
Discussion
The patient presented acute kidney injury (AKI) I according to laboratory results associated with countless coagulation times with decreased fibrinogen without platelet decrease, a picture compatible with L. obliqua poisoning according to the pathophysiological study of Alvarez Florez et al., in 2010. (6).
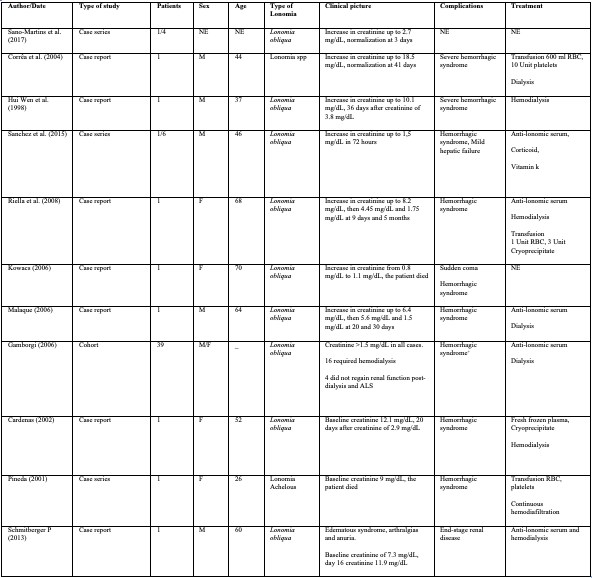
We found 1 cohort study and 10 case reports in which patients with lonomiasis developed acute kidney injury. In all cases there was alteration in the clotting times at the time of admission to the hospital. The patients presented hemorrhagic syndrome and AKI between 8 and 72 hours after contact with the venom (7), also hematuria and mucocutaneous bleeding. AKI was determined using the current creatinine elevation criteria (which were common to all cases). It should be noted that among the cases not selected for not presenting AKI, other potentially fatal bleeds were found, 9% of which were gastrointestinal, 4% intracranial, and 4% alveolar hemorrhage.
Among the cases in which AKI was documented, 9 were caused by L. obliqua, 1 by L. achelous, and one was not typified. These figures are consistent with the epidemiological data in the literature, which shows a higher incidence of serious clinical involvement due to L. obliqua venom in Colombia. Figures 2 and 3 (7).
Regarding the pathophysiology of the kidney injury, in most patients it was related to the hemodynamic changes secondary to the hemorrhagic syndrome. Among the most important changes are the decrease in systemic blood pressure and prerenal injury secondary to generalized renal ischemia due to a state of hypoperfusion (8). In addition, kidney local inflammatory processes caused by the larva venom toxins attribute an essential complementary factor to the renal involvement (3). A renal puncture with biopsy was performed in one of the reported cases and on one of the patients included in the cohort study. It showed acute tubular necrosis with mesangial proliferative glomerulonephritis and mononuclear interstitial infiltrates (9), and only signs of acute tubular necrosis (3), respectively, which supports the previous approach.
The case report and the theme review by Ávila et al., in 2013, and the Brazilian Ministry of Health agree that the patient involvement can be classified as mild, moderate or severe (7,8). According to this, all the cases reported and those included in the cohort study had a severe involvement due to the presence of AKI.
According to the creatinine elevation for the diagnosis of acute kidney injury, the recorded range ranged between 1.1 and 12.1 mg/dL. Regarding the AKIN classification for AKI, one of the patients was AKIN I, two cases corresponded to AKIN II, and seven were AKIN III. Within the reported cases, seven patients were taken to hemodialysis and one developed chronic kidney failure. Among the clinical and paraclinical characteristics for renal replacement therapy, it was observed that all patients had a creatinine level ≥4 mg/dL and clinical signs of uremia; 71% had persistent oligo-anuria for more than 48 hours, and 14% had hyperkalemia (potassium of 5.7 mEq/L). Of the 11 patients, 4 received Anti-Lonomic Serum (ALS). The duration of dialysis therapy in these patients did not exceed 20 days, and there was clinical and paraclinical improvement in the control performed the day after the administration of the serum, especially in the hematological parameters and the coagulation.
On the other hand, of the 2,067 patients included in the cohort study, 39 presented AKI, defined in this study as: serum creatinine greater than 1.5 mg/dL without a history of kidney disease. However, it is important to mention that the study was carried out in two different periods, because before 1995 there was no specific treatment for the lonomic accident; due to this, the initial management of the first 175 patients who entered the cohort was different. Of these, 5 developed AKI (2.9%). On the other hand, of the 1,892 patients who later entered the study, and who were treated with ALS, 34 had AKI. In both periods there were two patients who progressed to chronic kidney disease (10.3%), and there was only one fatal outcome due to AKI in the first period (3).
It is worth noting that this study suggests that the development of AKI is directly related to the amount of inoculum with which the patients had contact, and this, in turn, is proportionally linked to the number of caterpillars at the time of exposure. 90% of the patients with AKI recovered all or part of the renal function, and these cases were caused by L. obliqua (3).
Considering the case reports and the cohort study, the duration of dialysis therapy generally varied according to the decrease in serum creatinine values over time and the progressive increase of urine output; four days was the shortest time interval, and 45 days the longest.
In many cases, symptomatic treatment was given with corticosteroids and antihistamines, given the inflammatory and anaphylactic manifestations presented by the patients. However, the treatment for erucism consists of washing the affected area with cold water, applying cold compresses, infiltration with 2% lidocaine and topical corticosteroids (10).
Among the treatment alternatives for the moderate to severe involvement found in the literature and that were thought effective before ALS became available, are antifibrinolytic drugs (aprotinin, epsilon-aminocaproic acid and tranexamic acid), purified fibrinogen, cryoprecipitates, and replacement therapy (whole blood or fresh frozen plasma) (7,8). However, only a small benefit has been seen in the resolution of symptoms, but not a total reversal of the effects of the larva venom toxins (7).
With the production and implementation of ALS, not only a rapid and evident resolution of the clinical manifestations was observed, but a clear reversal of the effect of the venom toxins due to its main component, which is a heterologous immunoglobulin specific to the L. obliqua venom. (11), as long as the treatment was started within 48 hours after exposure to the venom (12). As previously mentioned, not all patients received ALS. However, there were no fatalities among those who received it. Instead there was a higher mortality in those who received alternative treatments, which increased when patients were exposed to the Obliqua species. The cause of death in these cases was secondary to the manifestations of the hemorrhagic syndrome, especially to intracranial hemorrhages (2).
Finally, regarding the prognosis of patients with lomonic accident associated with AKI, it has been seen that the degree of morbidity and mortality is related to the severity of the clinical involvement and the alterations in the paraclinical tests at the time of hospitalization (12). Regarding the associated kidney condition, the follow-up of some of these patients was done with periodic controls of serum creatinine levels to define the condition’s progression or improvement, given that some patients developed chronic kidney disease after the lomonic accident. Some of the risk factors identified in the cohort study were age, time elapsed before starting treatment, occupation, number of caterpillars involved, severity of bleeding and low platelet count. However, there are no conclusive studies on how the outpatient control of these patients at risk of developing chronic kidney disease should look like (3).
Pathophysiology
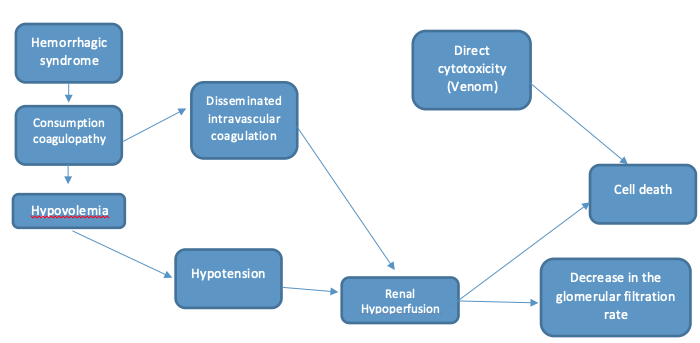
Envenomation by Lonomia spp., especially by L. obliqua, causes acute kidney failure through various mechanisms like massive deposition of microthrombi in glomeruli associated with disseminated intravascular coagulation, direct nephrotoxic action of the venom, hemodynamic changes, ischemic renal lesions, band acute tubular necrosis following the hemorrhagic shock (6), that will be describe below. Since the kidney is a highly vascularized organ whose function is to filter and excrete waste, including venom, it is very susceptible to toxins, with a variable spectrum of manifestations ranging from interstitial tubule nephritis, passing through glomerulonephritis, vasculitis, tubulointerstitial nephritis and acute tubular necrosis, the latter being the most frequent (13). Envenomation with this species causes what is known as severe hemorrhagic syndrome, which has clinical manifestations such as coagulation alterations, ecchymosis, acute kidney failure and generalized hemorrhage (2,9,13,14). The components with pro-coagulant, fibrinogenolytic, proteolytic and hemolytic activity cause a condition compatible with disseminated intravascular coagulation associated with a consumption coagulopathy, caused by a plasminogen activator, an enzyme similar to plasmin, two proteases that act on factor V and another on factor XIII, generating an activation in the fibrinolytic cascade that can mask the latter (4,15), which are one of the mechanisms that cause kidney damage (14,16,17) by forming microthrombi in the kidney microcirculation and generating a state of hypoxia and tissue ischemia that alters its functioning and causes cell death (6,3,14). On the other hand, the resulting hemolysis generates hemoglobin deposits in the renal tubules, and its degradation produces oxidative stress and endothelial damage (18).
However, the main mechanisms described for progression to acute kidney failure are the hemodynamic changes caused by the hemorrhagic syndrome, which decrease the mean arterial pressure, and therefore the renal perfusion pressure, which leads to a decrease in the glomerular filtration rate; hemolysis, which generates waste products that alter kidney function; alteration of vascular permeability, which causes edema, tubular obstruction and glomerular fibrin deposition, which contributes even more to tissue damage and becomes a postrenal etiology of this acute kidney failure; and last but not least, the direct nephrotoxicity of the venom produced by the action of metalloproteinases, serine proteinases, C-type lectin, phospholipases A2 and different oxidases (12,19,20).
A decrease in the glomerular filtration rate is observed, associated with the inability of renal tubules to absorb electrolytes due to the damage caused in the tubular epithelium; this alters the function of the Sodium-Potassium ATPase pump (21), initially increasing its function, which initially increases urine output and decreases urine osmolarity. After 6 hours of exposure to the venom, massive proteinuria is observed. Histologically, there is acute tubular necrosis with tubule dilatation, cytoplasmic vacuolization, degeneration and desquamation of the necrotic epithelium, which causes an intense inflammatory response with tissue fibrosis at 48-96 hours (12,14,17).
The kinin-kalicrein system (KKS) is activated by the kalicrein- and kininogenase-activating proteins present in the venom, which generates the release of bradikinins (pro-inflammatory substance) by means of low molecular weight kininogen (LMWK) and stimulating precalikrein in plasma (14); this leads to an increase in vascular and tissue permeability resulting in a decrease in blood pressure and edema in peripheral tissues (14,16,22). The KKS is important for the pathophysiological mechanism of renal failure, the kalikreina directly activates factor XII which generates an activation of Kinina for thrombus formation, by the intrinsic pathway (14,15). The effect of quinines is found in the bradykinin 1 (BR1) and bradykinin 2 (BR2) receptors, these receptors are involved in different processes such as inflammation, atherosclerosis, neuropathic pain and cerebrovascular disease; but in experimental models, when there is a blockage of the KKS, a decrease in flow, capillary leakage, inflammation and thrombi has been evidenced decreasing the deleterious effects of poisoning and with it the renal function (14). The venom components can be located in vascular smooth muscle cells (VSMC) in vivo and in vitro, this activation contribute to induce the formation and deposition of fibrin in the glomerular capillaries, and then reduce the filtration capacities, the L. obliqua venom can trigger reactive oxygen species (ROS) through the activation of NADPH oxidase favoring the coagulation cascade and incoagulability (14).
It has been seen that kidney tissue undergoes an inflammatory process caused by Lonomia obliqua venom, associated with a systemic inflammatory response whose main symptoms are pain and edema at the contact site (23,24) with a systemic production of TNF, IL8, IL6, histamine, prostaglandins, nitric oxide (NO) and other vasoactive inflammatory mediators or endothelial adhesion mediators. At kidney level there is also a series of inflammatory reactions; there is an increase in leukocyte migration and cell adhesion, production of IL8, TNF-α, ICAM-1, E-selectin and nitric oxide, and activation of COX-2, iNOS, NADPH oxidase (6 ,9,14,16,25,26); this produces oxygen reactant of mitochondrial origin, which further damages endothelial cells, thus inducing the adhesion and extravasation of all this inflammatory reaction, increased vascular permeability, glomerular dilatation, distention of Bowman’s space and interstitial edema. In addition, inhibition of metalloproteinases (MMP) has been observed, which produces deposits of collagen and other matrix proteins (14,24,27). The Figure 3 describe the physiopathology.
Diagnosis
The clinical record and physical examination are essential factors for an adequate diagnosis. Patients usually consult after a not very long period of evolution after the contact with the larva of L. obliqua (28,29), reporting a clinical picture of malaise associated with burning pain at the contact site, myalgia, and occasionally high temperature, which is usually accompanied by hemorrhagic systemic manifestations (gingivorrhagia, epistaxis, ecchymosis and even digestive hemorrhages) (8,15,17,30). In some very severe cases there may even be intracerebral hemorrhage. However, the prognosis is guarded, and this is the first cause of death in these patients (16). To make an adequate diagnosis, paraclinical tests must be performed; an essential test are the coagulation times, which are altered in all cases with an important prolongation, secondary to a hemorrhagic process with a fibrinolytic pathophysiological basis. Prothrombin time (PT) and partial thromboplastin time (PTT) values are usually variable, due to the initial characteristics of the patient’s coagulation system (10,11,31). However, it has been observed that the values in L. obliqua envenomation are always above the control, since the larva has two procoagulant proteins that affect the coagulation cascade in the common pathway, lopap prothrombin activator, and losac factor X activator; this is the reason why both PT and PTT are altered (13,32,33). It is possible to evaluate at the same time factors that are altered by wear, such as fibrinogen and platelets, and there is significant hypofibrinogenemia and thrombocytopenia secondary to the intrinsic fibrinolytic hemorrhagic process that develops with L. obliqua toxins (16,34). Normal volume anemia may also occur due to blood loss, which will be directly proportional to the patient’s clinical condition (34,35).
A kidney function test and a partial urinalysis should be performed, as in this case hematuria is a clinical manifestation of an intrinsic kidney disorder that is associated with the development of acute tubular necrosis in some patients, which is the basis of acute kidney failure, and therefore of the development of an unsatisfactory systemic state with high mortality rates; this factor alerts to the need of establishing a fast and effective therapeutic plan (9,28,36,37). The diagnosis of acute kidney failure was made in a study by Gambordi et al. (3), with creatinine results >1.5 md/dL in patients without a history of kidney failure (3); the study reported that, although the administration of ALS did not produce an immediate improvement in acute kidney failure, it did have an impact on the progression of the disease in terms of its severity and the prevention of chronic kidney disease (3). Another diagnostic method performed by Burdmann et al. (1996) was a renal biopsy 17 days after the caterpillar envenomation; the histopathology showed thickening of Bowman’s capsule and tubule atrophy; other findings in the renal biopsy were compatible with acute tubular necrosis in the recovery phase (3). However, coagulopathy makes it difficult to perform this study in the acute phase. Proteinuria was also observed in patients with acute kidney failure, which is greater in the first 6-12 hours after the contact (3).
Treatment
Initially, a symptomatic therapeutic approach can be effective in terms of reducing pain, normalizing temperature curves and reducing bleeding, with all the hemodynamic implications that this entails. For this reason, the use of NSAIDs, corticosteroids and first-generation antihistamines is indicated to decrease the inflammatory response and achieve an initial symptomatic stability (37).
As previously stated, the pathophysiology of L. obliqua envenomation consists of the development of consumption coagulopathy due to the inoculation of two procoagulant enzymes present in the larva venom, which generate a decompensated response by the fibrinolytic system. Therefore, antifibrinolytic agents do not produce a symptomatic or laboratory improvement; they inhibit the degradation of fibrin and do not produce an improvement in cases of coagulopathy in which the factors are consumed, as in the L. obliqua envenomation (15,38).
Conversely, in cases of L. achelous envenomation, the symptomatology is secondary to an abnormal and exaggerated fibrinolytic activation (39), so the use of antifibrinolytic drugs such as aminocaproic acid and tranexamic acid is recommended, in order to decrease the bleeding rate, which in some cases can be severe; this improves the patient’s hemodynamic conditions, preserving his stability and reducing the risk of developing acute kidney failure due to renal hypoperfusion with a subsequent decrease in the glomerular filtration rate (31,40). This implies a clinical and symptomatic improvement in cases of L. achelous envenomation.
However, in cases of L. obliqua envenomation, the larva toxins continue to cause systemic damage if the antidote or anti-lonomic serum, also known as “anti-venom”, is not administered (41); this antidote was developed for the first time in 1996 in Brazil, by extracting fragments of immunoglobulin G from previously immunized horses (38). The administration of this serum produces a significant reversal in the clinical condition of the patient envenomed by L. obliqua; a symptomatic and paraclinical improvement is observed, since it has been shown that a few hours after administering this serum, the clotting times are normalized, there is a substantial increase of the fibrinogen and in the platelet count, and optimal values are observed in kidney function tests; this is secondary to the increase in renal perfusion, with a subsequent increase in the glomerular filtration rate and a decrease in the nephrotoxicity produced by toxins inoculated by the larva-man contact (6,15,38).
In the same way, the use of antibiotics, specifically of first-generation cephalosporins, has been described to prevent or treat the concomitant development of skin and soft tissue infection (42). It is also recommended to give vitamin K to these patients, as this will enhance the action of vitamin K-dependent coagulation factors, which promotes an adequate homeostatic control (42).
Recently, in an experimental study in animals conducted by Berger et al. (14), they describe the inhibition of KKS by means of aprotinin as a complementary management to ALS or as an alternative to reduce the complications and effects of poisoning through the inhibition of receptor BR1 and BR2 (14).
Prognosis
Although epidemiologically significant morbidity and mortality rates have not been described in cases of L. obliqua envenomation, it has been found that the prognosis of the patients is directly related to the symptoms and the initial clinical and laboratory conditions with which the patient consults for the first time. The presence of intracerebral hemorrhage as a clinical manifestation of consumption coagulopathy has fatal outcomes, which is the first cause of death in these patients (16,36).
Similarly, patients with acute kidney failure as a manifestation of nephrotoxicity from the larva have hemodynamic and systemic repercussions that can quickly lead to multiorgan dysfunction (9,36). Intravascular hemolysis is a rare complication that has been observed only in the most severe cases of this pathology, associated with contact with a large number of caterpillars, and as well as the two aforementioned entities, it also has a worse prognosis in those patients who present them (43,44).
The patient’s degree of hemorrhage, and even more so his degree of shock, warns of the need for an adequate approach and optimal hydric resuscitation, as well as for the rapid administration of pro-hemostatic drugs, such as antifibrinolytic drugs and ALS (40).
Conclusions
Even though the most frequent clinical feature of the Lonomia envenomation is the alteration of clotting times, it has been found that acute kidney injury should not be disregarded as this condition increases the risk of further undesirable outcomes or even death.
The development of acute kidney injury is mediated by various mechanisms. These include the hypoxia and tissue ischemia caused by the haemodynamic changes and the microthrombi formation secondary to the haemorrhagic syndrome. Moreover, a proinflammatory state appears to affect the kidney locally, not only worsening its perfusion but also decreasing progressively the glomerular filtration rate and making it prone to develop acute tubular necrosis.
To perform an adequate diagnosis, some of the tests that should be done are the coagulation times, fibrinogen levels, an hemogram (searching for thrombocytopenia and anemia) in junction with the kidney function and urinalysis. Although some findings in the renal biopsy are helpful to confirm the diagnosis, this procedure is not feasible in the acute phase of the disease and could delay treatment.
A symptomatic approach for treatment is indicated to decrease pain and the inflammatory response. However, the definitive manage depends on the type of envenomation. For L. obliqua species, the anti-lomonic serum is the first line treatment to control promptly the haemorrhagic manifestations while for L. achelous, the antifibrinolytic agents may perform well.
Finally, the prognosis for these patients is not well described yet. Nonetheless, it is proportional to the severity of the clinical findings, the localization of the bleeding and the time of setting the treatment. In the case report, our patient had a good response to the treatment given as seen in his clinical and laboratory progression.