Introduction
The Americas region accounts for the third highest incidence of cervical cancer among the WHO regions, with more than 70.000 women diagnosed every year (1,2). Cervical cancer represents the first cause of cancer incidence in Bolivia and the second in 16 countries in the Americas. Regarding mortality, cervical cancer is the first cause of cancer mortality in 5 countries and the second cause in 13 countries in the region (1). Within the region, there are evident disparities, with an incidence three times higher in Latin America and the Caribbean than in North America (1).
Currently, 3.5 million people live with HIV in the Americas region, with an estimate of 740.000 women only from Latin America and the Caribbean (3). As mortality is rapidly decreasing due to the higher coverage of antiretroviral (ART) drugs, as well as a stagnant incidence rate, the Region is expected to have an increased number of women living with HIV (WLHIV) in the upcoming years. WLHIV are 4 to 10 times more prone to develop cervical cancer, and synergies among HPV and HIV infection has been elucidated (2,4,5). This represent a significant number of vulnerable women who can be disproportionally impacted by cervical cancer.
Previous systematic reviews and reports have evaluated the burden of cervical cancer and HPV infection amongst WLHIV (6, 7, 8, 9). However, representation of Latin American and Caribbean countries in such reviews has been limited and some recent data from cohort studies suggest significant differences between WLHIV populations from different world regions, not only in cancer incidence but also in associated HPV types and other factors such as exposure to ART (10,11).
Hence, to our knowledge no systematic review on the subject has been carried out for countries in the Americas Region. In this review, the Pan American Health Organization (PAHO) has commissioned the Centro Javeriano de Oncología at the Hospital Universitario San Ignacio in Colombia, to summarize the available data regarding cervical cancer epidemiology, prevention and early detection among WLHIV in Member States of the PAHO (12).
This systematic review is relevant considering the upcoming Global Strategy towards the elimination of cervical cancer as a public health problem, and the regional plans for cervical cancer control and prevention of HIV and sexually transmitted diseases (STDs) (13,14). The review will provide a situation analysis that will elucidate new and valuable information for policy makers for immediate action and will serve as a baseline for the Global Strategy and regional plans.
Methods
Aim and objectives
This systematic review aims to synthesize and report the existing knowledge about cervical cancer epidemiology, as well as access to screening and preventive services for women living with HIV in the Americas region.
This will be achieved by systematically searching, selecting and synthesizing the existing knowledge in order to answer our research questions. Therefore, the specific objectives are:
Summarize data on cervical cancer incidence and mortality among WLHIV in the Americas region.
Summarize data on prevalence of precancerous lesions among WLHIV in the Americas region.
Summarize data on prevalence of HPV infection among WLHIV in the Americas region.
Identify and characterize access to screening and preventive services around cervical cancer among WLHIV in the Americas.
Study design
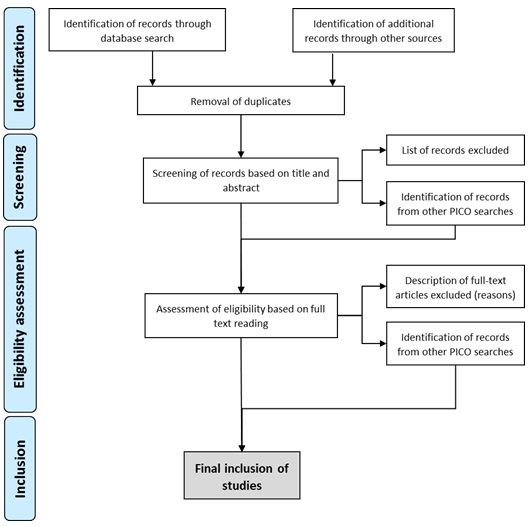
We designed the protocol in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) (Figure 1).
Search strategy
We will systematically search in PubMed via Medline and LILACS databases, and after that, we will conduct updates and searches for grey literature via TRIP-database and Google Scholar. Additionally, cross-referencing will be used to find additional articles for review.
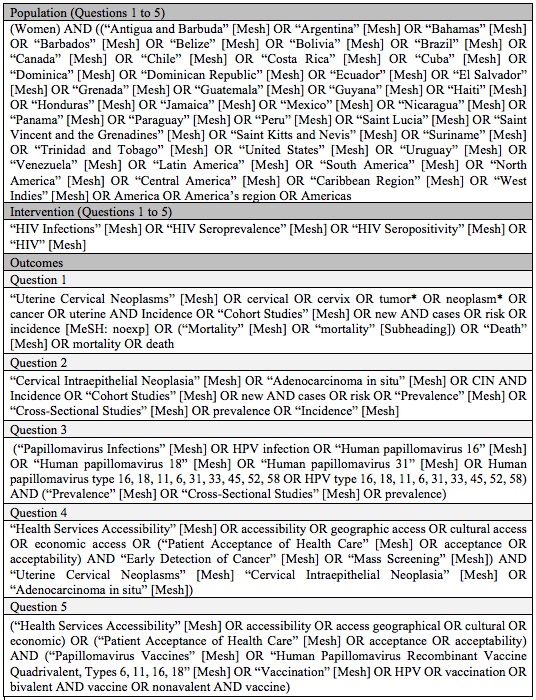
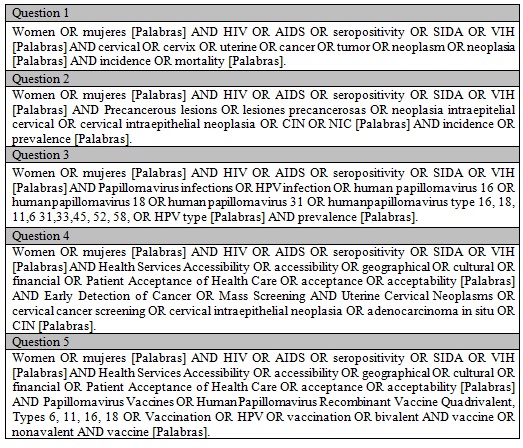
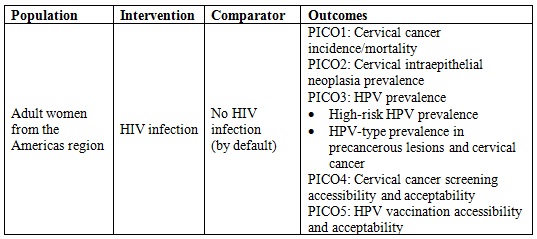
Five PICO questions were defined to carry out a structured search as indicated in Table 1. The terms used for every PICO are presented in the supplementary material (Appendix 1). Accessibility has been defined in three dimensions: physical accessibility, financial affordability, and acceptability (16). These dimensions recall patients and population perspectives beyond the availability and adequacy of health services. For our review, acceptability will be approached in three dimensions: geographic accessibility, economic accessibility, and cultural accessibility.
Table 1 PICO questions for the systematic searchHIV: Human Immunodeficiency Virus, HPV: Human Papillomavirus.
We will remove duplicates, and publications will be independently screened based on title and abstract by two members of the research team, and all disagreements will be solved by consensus.
Since a single study could report outcomes for different PICO questions, we will identify and label such studies for their inclusion regarding the corresponding question and outcome. All studies identified through this approach should follow general screening and evaluation procedures as described in the methodology.
Eligibility criteria
We will select papers based on the following criteria:
No language restriction.
No publication date range defined.
Full text availability.
Studies including adult women. Studies only on adolescents or men will be excluded.
Studies conducted in populations from PAHO affiliated countries.
Outcome specified and data on outcomes available as defined in the PICO questions.
For PICOs 1 to 3, the age as major determinant should be specified (either average age, median age, or age range). Studies with no overall age specified but age groups defined will be included if reporting outcome data at least for one age group.
Studies conducted in the general target population (adult women living with HIV). Studies in special populations excluded.
-
Type of study:
Quality appraisal
For all included studies, two independent reviewers will assess the methodological quality and risk of bias. For cross-sectional, descriptive and population-based studies, we will use the 20-item appraisal tool for cross-sectional studies (AXIS) (17). Cohort studies will be assessed using the checklist for cohort studies developed by the Scottish Intercollegiate Guidelines Network - SIGN (18). For qualitative studies, we will apply the “Joanna Briggs Institute Critical Appraisal Checklist for critical and interpretive research” (19).
Data management
Studies retrieved from the search will be listed in Zotero® to identify duplicates. If a given study is published in different journals but with differences in sub-group analyses or presenting updated data, we will extract data from all reports but include in the analysis only the latest data available. If related publications presenting different information are found, they will be included in the analysis indicating that they correspond to a group of reports with the same origin (cluster).
We will prepare three data extraction sheets in Microsoft Excel® for every PICO question: one to register eligibility criteria for all studies selected for full text review, the second for detailed data extraction from studies finally included in the review, and the third to register the quality appraisal in detail.
From each study included, we will extract general information (author, title, year of publication, observation period, database source of the study, type of study, and country). We will also extract data on the methods, including sample size, follow-up (cohort studies), eligibility criteria for the study population, age (range, median, mean), and statistics used for significance analysis. Finally, the outcomes for every PICO question will be extracted as absolute numbers when available in addition to the corresponding indicator (incidence rate, prevalence rate, percentage, etc.).
Data analysis
A separate report for every PICO question will provided. We will present data considering quality of studies and geographic representation (North America and Latin America and the Caribbean), including differences in outcomes and variability in studies.
Depending upon the results, we will also conduct meta-analyses. We will use Revman5 (Cochrane Collaboration, London, United Kingdom) to prepare our review and possible meta-analysis. For this, we will review heterogeneity and quality of studies in addition to availability of detailed information, particularly on age. We will apply a fixed effects model if the heterogeneity is small and a random effects model if the heterogeneity is high. Variation in values by geographic area, mean age and type of study are expected. We will use funnel plots (Christmas tree and “trim and fill”) to assess possible publication bias. Since these methods are unreliable if the number of studies is less than 10, we will only apply it if there are more than 10 studies available.
If no detailed information on age is found, we will contact the corresponding author to retrieve additional data for the analysis. Age group of relevance for the analysis in PICO questions 1 to 3 will be women under 20 years old, women 20 to 29, women 30 to 49, and women over 50 years old.
Discussion
HPV screening and HPV vaccination are the core for cervical cancer elimination globally and regionally (2). Settings with high HIV prevalence have some of the highest cervical cancer incidence rates; thus, the WHO plan highlights the need for greater effort to achieve elimination in such settings.
Previous reviews have included mainly African, European and North American countries (6, 7, 8, 20, 21, 22) with scarce representation of Latin America and the Caribbean (LAC), which correspond to the region with the second cervical cancer incidence among WHO Regions. To our knowledge, this will be the first review aimed at characterizing epidemiology and preventive activities around cervical cancer among WLHIV in the Americas, and particularly in LAC, a region with high disparities in access to health care.
Our search is designed to ensure the representation and inclusion of all countries in the region. We anticipate finding a large number of papers that might have been disregarded in previous reviews thanks to the use of a Latin American search engine and the inclusion of studies in other languages such as Portuguese and Spanish (6, 7, 8). We established our clinical questions to provide the most comprehensive evidence of the current situation of cervical cancer disease burden, high-grade precancerous lesions and HR-HPV prevalence in Women Living with HIV in the Americas, as well as the social response to HPV vaccination and cervical cancer screening.
Since we have developed several clinical questions, the final report will be divided into 3 main categories: cervical cancer and high-grade precancerous lesions prevalence (disease), HPV infection, and social response. This will allow a more in-depth statistical and subgroups analysis, per type of study. For outcomes related to the disease and HPV infection, we will assess HIV-related factors such as CD4+ cell count and use of antiretroviral therapy amongst studies providing in-depth analysis on the subject, which has not been covered for the Americas region. (6, 7, 8).
The lack of systematized data on WLHIV and cervical cancer epidemiology and related factors, will difficult the regional efforts towards the control and further elimination of cervical cancer. Our search is targeted to identify all publications on cervical cancer burden and related factors among WLHIV. In this review, the use of a Latin American search engine and the inclusion of studies in other languages, will allow us to identify more papers that may have been disregarded in previous reviews (6, 7, 8).
Conclusion
It is paramount to carry out a systematic review and summarize available data on the subject, seeking to set a baseline and provide new and valuable information for policy makers. Our search is targeted to identify all published evidence on cervical cancer burden and HPV infection among WLHIV in the Americas Region.