Introduction
On average, menopause occurs at 51 years of age (with ethnic and regional variations) (1). In Colombia, the age of onset is around 48.1 ± 5.39 years (2). The cessation of ovarian function leads to the appearance of vasomotor, psychological, somatic, and atrophic symptoms in estrogen-dependent tissues. Such physiological and pathological changes can harm the quality of life of postmenopausal women and even lead to serious physical and mental illness (3).
The symptoms of menopause are varied, depending on the physical, mental, social, cultural, and racial characteristics of each woman (2,4). It is estimated that 85% of women report at least one symptom of menopause, but only 10% seek medical attention (5). In menopause, vasomotor symptoms (hot flashes or night sweats), sleep disturbances, and vaginal dryness stand out (6).
Hormone replacement therapy (HRT) is the main indication for the treatment of estrogen deficiency (vasomotor symptoms, symptoms of urogenital atrophy, postmenopausal osteopenia, and osteoporosis), to improve the quality of life of women (7). Treatment should be individualized according to the clinical conditions of each patient, and hormonal and non-hormonal treatment can be offered (7,8).
Before the initiation of HRT, a thorough physical examination is recommended at the consultation to consider both indications and contraindications; therefore, it is necessary to record the history, physical examination, and other complementary tests (9,10).
Tibolone is a synthetic steroid, a selective regulator of tissue estrogenic activity, which controls menopausal symptoms (11), inhibits bone loss (12), and increases vaginal blood flow (13). After ingestion, it is converted into three active metabolites: 3α- and 3β-OH tibolone, which act on estrogen receptors in the vagina, bone, brain, and vascular wall, and the ∆4 tibolone isomer, which binds to androgen and progesterone receptors, whose androgenic action is primarily exerted in the liver and brain (14).
The Menopause Rating Scale (MRS) is a useful instrument to measure the severity of menopause-related symptoms and thus evaluate the quality of life (15). It is composed of three dimensions: a) somatic-vegetative, b) psychological, and c) urogenital, where eleven symptoms are grouped (15,16).
The Female Sexual Function Index (FSFI) is a reliable instrument to evaluate the sexual function of women in a wide age range, with adequate reliability and psychometric properties (17).
Given the scarce research in the Colombian population on the effectiveness of tibolone in the treatment of climacteric syndrome and sexual function in postmenopausal women (a stage that begins from the year of the last menstrual period until the end of life) (2,6,9), and given that no studies have been published on this subject at the regional level, the objective of this research was to establish the effectiveness of tibolone and the incidence of adverse effects in postmenopausal women.
Materials and methods
Design and population
A quasi-experimental, before-after study was conducted, which included sexually active postmenopausal women (natural menopause) over 40 years of age, with climacteric symptoms and last menstrual period at least 2 years before, with a stable partner and serum follicle-stimulating hormone (FSH) ≥40 IU/L and estradiol ≤25 pg/ml. This, in the period between January 1, 2012, and December 31, 2015, in a private clinic in Armenia (Quindío), a high complexity, referral center, which serves population affiliated to the subsidized regime by the State and the contributory regime, in the General Social Security System in Colombia. Women with any history of cancer, personal history of deep vein thrombosis, history of hysterectomy or salpingo-oophorectomy, liver disease (acute or chronic), mental illness, altered renal function, abnormal uterine bleeding of unknown etiology, psychopathological or social situation that would make it difficult to understand the instrument, change of department of residence, possible withdrawal from the study before six months of follow-up or difficulties for follow-up, having received HRT in the last six months and presenting alterations in transvaginal ultrasonography or with an endometrial line ≥4.5 mm were excluded.
The sample was calculated from the simple random sampling formula for an expected proportion of 50 %, with a confidence level of 95 % (α = 0.05) and β risk of 0.2 for a precision of ±0.15. According to these criteria, the sample size was 85 women, but it was decided to include the largest number of participants in the "Climacteric and menopause" program. Simple random sampling was performed using a table of random numbers.
Method
rom the consultation database of the clinic's "Climacteric and menopause" program, women candidates for participation were identified. The selection was made by identifying code N95.1 of the International Classification of Diseases: female menopausal and climacteric states. They were contacted by the research team made up of the principal investigator, a general practitioner, and a nurse practitioner, who verified that they met the inclusion and exclusion criteria. The objective of the study was explained to the selected women and they were asked to sign the informed consent form authorizing their inclusion in the research. Those who agreed to be part of the research were given a questionnaire to establish their sociodemographic and clinical characteristics, to subsequently apply the IFSF instrument and the MRS scale.
The IFSF is a questionnaire consisting of 19 questions that assess female sexual function in the last four weeks. It groups six domains: Desire (items 1-2), Excitement (items 3-6), Lubrication (items 7-10), Orgasm (items 11-13), Satisfaction (items 14-16) and Pain (items 17-19). Each question has between 5 and 6 options, with points assigned from 0 to 5. The score for each domain is multiplied by a factor. The result is the arithmetic sum of the domains: the higher the score, the better the sexuality. The total score ranges from 2 to 36; a score ≤26.55, or when any domain is less than 3.6 points, is considered as a criterion for risk of sexual dysfunction (17-19).
The MRS scale self-assesses menopausal symptoms. It is composed of three dimensions or domains: a) somatic, b) psychological and c) urogenital. The somatic domain evaluates four symptoms (hot flashes, cardiac discomfort, sleep disorders, and musculoskeletal discomfort); the psychological domain, four (depressive state, irritability, anxiety, and fatigue), and the urogenital domain, three (sexual problems, bladder problems, and vaginal dryness), for a total of eleven items. To each item the woman gives a value from 0 to 4 (0 = absent; 1 = mild; 2 = moderate; 3 = severe; 4 = very severe). The score of a domain corresponds to the sum of each item of that subscale; thus, the total MRS score will be the sum of the scores obtained for each domain. The higher the score obtained, the greater the deterioration in the quality of life (15,16,20). It is a reliable scale with Cronbach's α coefficient of 0.80. The internal consistency of each dimension varies between 0.60 and 0.87 (15,21).
All patients were examined by the principal investigator, who evaluated the presence of menopausal symptomatology (symptoms and signs) reported by the participants. The diagnosis of menopause was made with the finding of absence of menstruation for 12 months or more.
All participants were asked to undergo complementary tests: blood count, blood glucose, glycosylated hemoglobin (HbA1c), lipid profile (total cholesterol, HDL-cholesterol, LDL-cholesterol, triglycerides), thyroid-stimulating hormone (TSH), uroanalysis, creatinine, and liver profile (aspartate aminotransferase or glutamic-oxaloacetic transaminase and alanine aminotransferase or glutamate pyruvate transaminase, bilirubins and alkaline phosphatase). These were requested annually until the end of the study; as well as in those with hyperlipidemia, every 6 months. Samples of antithrombin III, plasminogen, tissue plasminogen activator inhibitor, and plasminogen activator inhibitor-1 were taken. Likewise, transvaginal ultrasonography (to evaluate endometrial polyps, pelvic masses, and endometrial hyperplasia; endometrial thickness ≥4.5 mm was taken into account to establish the presumptive diagnosis of endometrial abnormalities), cervicovaginal cytology, and mammography.
The nature of their clinical condition was explained to each patient and tibolone was offered as a therapeutic alternative, at a dose of 2.5 mg orally (every night at bedtime). Five follow-ups were performed: at one, two, three, four, and five years after starting therapy. At each follow-up, the presence and severity of symptomatology were evaluated using the MRS scale, and sexual function was evaluated using the IFSF; in addition, the appearance and nature of adverse effects were investigated. During the follow-up, the absence or decrease in the severity of the symptomatology was taken into consideration to evaluate the therapeutic effectiveness. The findings of each woman and the results of the MRS and IFSF scales were recorded in each control, both in the clinical history and in the form designed by the investigators.
Two auxiliary nurses were in charge of reviewing and taking the data from the clinical history, verified by a professional nurse (specialist in Health Auditing). Each participant's information was recorded on a specifically designed form, which was then entered into a database in Excel 14.0 Office® 2011, and sent to the main epidemiological team of the study.
Measured variables
Age, race, educational level, socioeconomic stratum, marital status, occupation, religious condition, origin, affiliation to the General Social Security Health System; height, weight, Body Mass Index (BMI); habits (alcohol intake, smoking, coffee consumption, addiction to psychoactive substances, sedentary lifestyle); sexual and reproductive health variables (age at menarche, age at menopause, evolution of menopause time, history of sexual abuse or sexual violence in marriage, time of cohabitation as a couple and sexual dysfunction in the couple); pathological history, sexual behavior: age of onset of coital sexual activity, sexual activity (masturbation, oral sex, vaginal or anal intercourse, frequency of monthly coital intercourse, number of sexual partners and time living together as a couple). Also, the presence and severity of symptomatology (symptoms and signs) of menopause; adverse effects of tibolone, and the domains of the IFSF instrument and the MRS scale. Endometrial ultrasonography, blood pressure, and laboratories were measured at each control.
Statistical analysis
Qualitative variables are described in absolute frequencies and proportions; quantitative variables are described in measures of central tendency (mean or median) and dispersion (standard deviation or range), as applicable. The cumulative incidence of adverse effects at the end of the study was established. To compare the before-after results, the χ² test was used for qualitative variables, and for quantitative variables, the Student t-test (for normal variables) or the Man-Whitney U test for variables with non-normal distribution or non-homogeneous variances. The analysis was performed with the Epi Info® 7.2 program. Data validation was performed in the Stata® 16.1 program. A value of p < 0.05 was considered statistically significant.
Ethical Aspects
The study was approved by the Ethics and Research Committee of the Clinic (Act 102 of 2011) and complied with the requirements for medical research on human subjects established in the Declaration of Helsinki and Resolution 8430 of 1993, which establishes the scientific, technical, and administrative standards for health research. All participants signed the informed consent to enter the study and were guaranteed the confidentiality of the information.
Results
From a population of 325 eligible women, 103 did not meet the inclusion criteria, leaving 222 as the population for randomization, so the study was done with 127 (57.2 %) women for the final analysis.
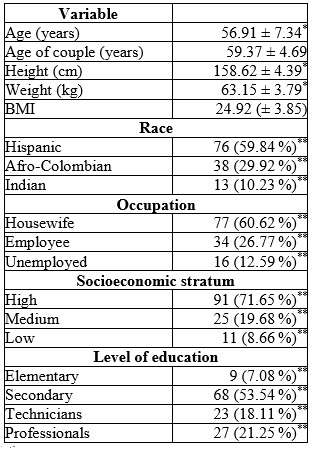
The mean age of the participants was 56.91 ± 7.34 years (minimum 42 and maximum 72 years). A total of 71.65% were married or living in a common-law relationship. A total of 84.25 % were of urban origin and 77.95 % belonged to the contributory insurance regime. A total of 89.76% professed the Christian faith. Table 1 describes the sociodemographic characteristics of the population.
Table 1 Sociodemographic characteristics of women users of tibolone in Armenia (Colombia), 2012-2015 (n = 127)
*Mean and standard deviation
**Absolute and relative frequency.
Concerning smoking habits, 18.11% smoked, with a median consumption of 3 cigarettes per day (range between 3 and 9). 92.91 % drank coffee; 74.8 % reported alcohol consumption, and 6.29 % reported the use of psychoactive substances. A sedentary lifestyle was detected in 69.29%.
The mean age at menarche was 11.79 ± 2.83 years. The mean age at menopause was 47.85 ± 3.98 years, with a mean duration of menopause of 9.52 ± 6.71 years. A total of 4.72% had received HRT in the last 3 years before the study.
In the pathological history, 36.22 % had osteoarthritis, 33.07 % had arterial hypertension, 41.73 % had dyslipidemias (56.6 % high triglycerides and 43.39 % high total cholesterol, with 47.16 % low HDL-cholesterol), 14.96 % had hypothyroidism, 8.66 % had type 2 diabetes, and 3.14 % had rheumatoid arthritis. A total of 5.51% were morbidly obese.
Regarding sexual behavior variables, the mean age of sexual activity initiation was 15.28 ± 4.37 years. Vaginal intercourse was practiced by 100% while anal intercourse was reported by 11.81%. Masturbation appeared as an uncommon experience (14.17 %). Oral sex was the sexual practice preferred by 86.61%. The frequency of monthly sexual relations (last 30 days before the interview) showed a median of 2 (range between 1 and 4). The length of time living together as a couple was 13.98 ± 7.52 years. The median number of sexual partners reported 3 (range between 1 and ≥9).
A history of sexual abuse was reported by 18.89% of the participants while sexual violence in marriage was reported by 9.44%. A total of 56.69% of the participants stated that their partners had some sexual dysfunction.
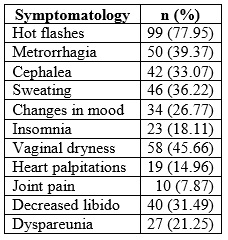
The menopausal symptoms mentioned by the patients, in order of frequency, are detailed in Table 2, and among them the most frequent were: hot flashes (77.95 %), followed by fatigue/fatigue (62.99 %) and vaginal dryness (48.03 %). 75.59 % presented 3 or fewer symptoms; 15.74 %, 4, and 8.66 %, 5 or more symptoms, with a median of 3 (range between 1 and ≥6).
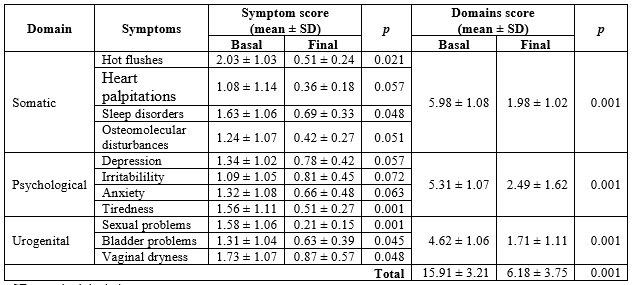
The MRS score is presented in Table 3. The symptoms showing the greatest severity were those of the somatic domain, reporting an average of 5.98 ± 1.08 points. The one with the least symptomatic impact was the urogenital domain, with 4.62 ± 1.06 points. According to symptom severity, 18.11 % (n = 23/127) of the women were found to have "mild" symptoms; 22.83 % (n = 29/127), "moderate"; 27.55 % (n = 35/127), "severe"; and 31.49 % (n = 40/127), "very severe". According to severe symptomatology, 52.5 % were somatic, 27.5 % psychological and 20 % urogenital. At the beginning of the study (baseline), the value of the MRS score was 15.91 ± 3.21 and at the end, it was lower, with a value of 6.18 ± 3.75; the statistically significant difference (p = 0.001). At the beginning of the study (baseline) in the somatic domain, the MRS value was 5.98 ± 1.08 and at the end, it was lower, with a value of 1.98 ± 1.02; statistically significant difference (p = 0.001).
From the first month of treatment, the effectiveness of tibolone began to be noted: 57.48% (n = 73/4127) of the patients reported an improvement in symptoms, which was progressive throughout the first year and remained constant until the end of the study (Table 3). Vaginal dryness persisted in 7.07% (n = 9/127), and dyspareunia in 6.29% (n = 8/127). At the end of the study, none of the women rated the severity of symptomatology as "severe" or "mild", giving as a result an improvement in menopausal symptomatology of 94.48 % (p < 0.05) while 5.51% persisted with "moderate" symptoms (p > 0.05).
Tabla 3 Menopause Rating Scale (MRS) score in postmenopausal women in Armenia (Colombia), 2012-2015 (n = 127)
SD:standard deviation.
At the beginning of the study, the mean IFSF score was 23.19 ± 6.41 points (lower limit = 8.57 and upper = 29.38). At the end, the mean score reached 28.02 ± 6.63 points (lower limit = 10.52 and upper = 32.75), with statistically significant difference (p = 0.001). A 52.75 % of sexual dysfunctions were reported. It was found that 45.66 % presented 2, and 23.62 %, 3 or more concomitant sexual dysfunctions, with a median of 2 sexual dysfunctions per woman (range between 1 and ≥4). Hypoactive sexual desire was present in 31.49 % of the participants. When contrasting the IFSF domains between the measurement before therapy and at the end, a score of 3.47 ± 1.06 vs. 4.62 ± 1.17 (p < 0.05) was observed in the desire domain. Regarding lubrication, the score was 3.52 ± 1.07 vs. 4.74 ± 1.05 (p < 0.05) (Table 4).
Table 4 Female sexual function index in postmenopausal women in Armenia (Colombia), 2012-2015 (n = 127)
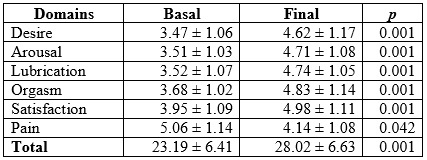
At the end of the investigation, it was found that sexual dysfunctions decreased to 7.08%, resulting in an improvement of 86.56%. On the other hand, satisfactory sexual encounters were reported by 21.25% compared to 82.67% at the end (p < 0.05). At the 5-year follow-up, 92.91% (n = 118/127) reported an increase in vaginal coital frequency, with a median of 4 monthly encounters (range between 3 and 9).
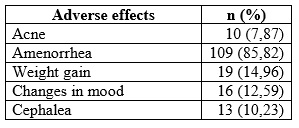
Adverse effects (AE) were present from the first 3 months after initiation of therapy. The most frequent were amenorrhea (85.82%), mastalgia (18.89%), and edema (17.32%). The total AEs reported and their frequency is described in Table 5. In most women, the AEs occurred more frequently between the first and third month of therapy, decreasing spontaneously and progressively at the end of one year; most were mild, tolerable, and transitory.
Table 5 Adverse effects of tibolone in postmenopausal women in Armenia (Colombia), 2012-2015 (n = 127)
In the metabolic profile at baseline, we found a mean fasting blood glucose of 109.58 ± 37.62 mg/dl and HbA1c: 7.13 ± 1.48 with a slight reduction of 6 % at the end of the study (97.16 ± 35.29 mg/dl and HbA1c: 6.78 ± 1.39; p > 0.05). There were no significant changes in transaminases, bilirubins, alkaline phosphatase, creatinine, hemogram, or TSH (p > 0.05).
In relation to the lipid profile, at the end, a favorable result was observed with a 15 % reduction in triglycerides (235.67 ± 61.8 vs. 201.95 ± 69.7 mg/dl; p < 0.001), a 20 % reduction in total cholesterol (239.67 ± 58.62 vs. 192.78 ± 46.53 mg/dl; p < 0.001), 20 % in LDL (145.37 ± 38.62 vs. 116.87 ± 32.43 mg/dl; p < 0.001), 12 % in VLDL (32.87 ± 12.56 vs. 29.14 ± 10.25 mg/dl; p > 0.001), and 9 % in HDL (59.26 ± 13.81 vs. 54.31 ± 12.75; p > 0.001).
In the analysis of fibrinolysis and coagulation, tibolone was found to reduce tissue plasminogen activator inhibitor and plasminogen activator inhibitor-1 by 15% to 20 % (1.83 ± 1.02 vs. 1.05 ± 0.96 ng/ml; p < 0.001, and 45.87 ± 16.23 vs. 37.95 ± 10.98 ng/ml; p < 0.001, respectively). Fibrinogen decreased 30 % (285.72 ± 59.37 vs. 201.48 ± 41.52 mg/dl; p > 0.001), and plasminogen, 15 % (19.32 ± 4.71 vs. 15.78 ± 3.57 mg/dl; p < 0.001). An increase in antithrombin III of 20 % (87 % vs. 105 %; p < 0.001) was reported. The changes were present from the first year of follow-up and were maintained until the end of the study.
In blood pressure figures, a 7% decrease was observed in mean systolic pressure (128.57 ± 14.39 vs. 120.9 ± 13.58 mmHg; p > 0.05) and diastolic blood pressure (87.51 ± 10.57 vs. 81.39 mmHg; p > 0.05).
The endometrial line went from 4.13 ± 0.52 mm at baseline to 4.06 ± 0.47 mm at the end of the study, a difference that was not statistically significant (p = 0.372). Cervico-vaginal cytology and mammography showed no alterations throughout the study.
The average weight gain was 1.52 kg/year, present in 74.01% (n = 94/127) of the total participants; while 12.59% (n = 16/127) recorded a decrease of 0.76 kg/year.
No serious AE (heart attacks, venous thrombosis or pulmonary embolism, endometrial, or breast cancer, fractures, cardiovascular disease or deaths during follow-up) were reported.
At the end of the study, 95.27% of the total number of women participants were satisfied with the effectiveness of tibolone.
Discussion
In the present investigation, an improvement in menopausal symptomatology of 94.48 % (p < 0.05) was observed; while sexual dysfunctions reported an improvement of 86.56 %. A positive impact on sexual desire, frequency, and satisfaction was observed. Although adverse effects were frequent, they were mild, tolerable and transient, and resolved spontaneously. The results are similar to those reported in the TOTAL (TOlerabilit Trial Comparing Activelle with Livial) study by Hammar et al. (22), a multicenter study (conducted in 32 centers in 7 European countries, in 572 women), which showed that tibolone reduces menopausal symptoms to a similar degree as continuous combined HRT, but produces less bleeding and breast tension. Likewise, he LISA study (Livial International Study in Sexual Arousal Disorders), a multicenter, double-blind, randomized clinical trial (with 403 women) showing that both tibolone and E2/NETA resulted in improved sexual function overall, with increased frequency of sexual events and reduced personal distress related to sexuality; but scores were higher on the IFSF domains in the tibolone group (p < 0.05) (23).
The increase in sexual desire induced by tibolone may be due to the actions of estrogens and androgens associated with their metabolites, as well as to the low activity of sex hormone transporting globulin (24), which correlate with both increased serum estradiol and free testosterone concentrations (25).
In the results of a randomized, controlled study (LIBERATE), which included 3148 women with climacteric symptoms and whose objective was to demonstrate the non-inferiority of tibolone versus placebo concerning the risk of recurrence in patients with breast cancer, after 3.1 years of follow-up no differences were found between the two groups regarding mortality, cardiovascular events, and the appearance of other gynecological cancers. Vasomotor symptoms and bone mineral density improved significantly with tibolone; however, breast cancer recurrence was higher than in the placebo group (15.2% vs. 10.7%; HR: 1.40; 95% CI: 1.14-1.70; p = 0.001). The interpretation of the study is that tibolone increases the risk of recurrence in breast cancer patients while alleviating vasomotor symptoms and preventing bone loss (26).
Regarding the lipid profile, our data are similar to those presented by Bjarnason et al. (27), in a double-blind, randomized, placebo-controlled study (2 years, conducted in 91 healthy postmenopausal women), which showed a reduction in total cholesterol and triglycerides.
In THEBES (Tibolone Histology of Endometrium and Breast Endpoints Study), a multicenter, randomized, double-blind study of 3240 women, designed to address conflicting reports in the literature on the endometrial safety of tibolone, previous findings that it does not induce endometrial hyperplasia or carcinoma in postmenopausal women and is associated with a better vaginal bleeding profile than conventional HRT were confirmed (28). However, in a randomized study, where 4538 women, aged between 60 and 85 years, were assigned during a median of 34 months of treatment, the tibolone group reduced the risk of fracture, breast cancer, and possibly colon cancer; but increased the risk of stroke in older women with osteoporosis (29). From this follows the idea that tibolone is the HRT in women under 60 years of age, in whom the risk of thrombosis is low.
It is important to clarify that tibolone is not anti-estrogenic nor does it inhibit aromatase; therefore, its mechanism of mammary protection is explained by enzymatic actions by inhibiting sulfatase and 17ß-hydroxysteroid dehydrogenase and stimulating sulfotransferase to increase the production of inactive sulfates (30). In turn, metabolites do not have the same action on sulfatase activity in all tissues, as they only moderately inhibit sulfatase in the endometrium and do not provide inhibition in bone (allowing for a greater estrogenic impact) (31). Estrogenic influence on the vagina alleviates vaginal dryness and dyspareunia, which is associated with improved sexual response and increased libido (14,32,33).
The main strength of this research is that it establishes the results of one of the most prescribed HRT, but little evaluated in the Colombian population. This makes it easier to know its impact on climacteric syndrome and sexual function in women who are in the "window of opportunity" period (9,14). Another strength is the use of validated instruments and the study period. Additionally, it is the first of its kind to be conducted in Colombia. Among the limitations is the small sample size, due to the characteristics of the participating population, since it has a high level of selection, which is why only 127 women were analyzed, although it was enough to complete the expected number during the study period. On the other hand, being a quasi-experimental research, its lower precision and validity, compared to experimental designs, is understood. There is a need for studies of higher methodological quality to evaluate the effectiveness of tibolone on menopausal symptoms and sexual function, as well as its impact (safety and tolerability) in women over 60 years of age.
Conclusions
The administration of tibolone has a positive influence on the treatment of climacteric symptomatology, since it reduces both its quantity and severity, with a clear improvement in sexual function, with mild, tolerable and transitory adverse effects. Randomized, double-blind, controlled clinical trials on its efficacy and safety in older and larger populations are needed. Future research should confirm the findings described.











 text in
text in