Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
International Journal of Psychological Research
Print version ISSN 2011-2084
int.j.psychol.res. vol.6 no.spe Medellín Oct. 2013
Sex differences in gray and white matter structure in age-matched unrelated males and females and opposite-sex siblings.
Diferencias sexuales en la estructura de la materia gris y blanca en hombres y mujeres de la misma edad y hermanos de distintos sexos.
Anouk den Brabera, Dennis van ‘t Enta,*,**, Diederick Stoffersb, Klaus Linkenkaer-Hansenc, Dorret I. Boomsmaa and Eco J.C. de Geusa
a Department of Biological Psychology, VU University Amsterdam, Amsterdam, The Netherlands.
b Department of Sleep and Cognition, Netherlands Institute for Neuroscience, Amsterdam, The Netherlands.
c Department of Integrative Neurophysiology, Center for Neurogenomics and Cognitive Research, Neuroscience Campus Amsterdam, VU University, Amsterdam, The Netherlands.
* Anouk den Braberand Dennis van ’t Ent contributed equally to this manuscript.
** Corresponding author. Address: van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands; Tel.: +31 20 5982534; fax +31 20 5988832. E-mail address: d.vant.ent@vu.nl
Received: 02-08-2013-Revised: 11-09-2013-Accepted: 23-09-2013
ABSTRACT
Apart from the general finding of larger global brain volumes in men, neuroimaging studies that compared brain structure between men and women have yielded some inconsistencies with regard to regional differences. One confound when comparing men and women may be differences in their genetic and or family background. A design that addresses such confounds compares brain structures between brothers and sisters, who share their genetic and family background.
In the present study, we aimed to contribute to the existing literature on structural brain sex differences by comparing regional gray and white matter volume, using voxel based morphometry (VBM); and white matter microstructure, using tract-based spatial statistics (TBSS), between 40 unrelated males and females, and contrasting the results with those obtained in a group of 47 opposite-sex siblings, including 42 dizygotic opposite-sex (DOS) twin pairs.”
Our results showed that men had larger global brain volumes as well as higher mean fractional anisotropy across the brain and showed regionally enlarged gray matter volume and higher fractional anisotropy in, or surrounding, subcortical structures (hypothalamus, thalamus, putamen and globus pallidus and rostral midbrain). Increased gray matter volume in women was restricted to areas of the cortex, including inferior temporal, insular, cingulate, precentral and frontal/prefrontal regions.
These sex differences were generally consistent between the unrelated male-female pairs and the opposite-sex sibling pairs. Therefore, we conclude that these sex differences are not the result of confounding differences in genetic or family background and that the etiology of these sex differences merits further investigation.
Key Words: Sex differences, opposite-sex twins, structural magnetic resonance imaging, voxel based morphometry, diffusion tensor imaging, fractional anisotropy.
RESUMEN
Aparte del descubrimiento general de volúmenes cerebrales globales mayores en hombres, los estudios de neuroimagen que han comparado la estructura cerebral entre hombre y mujeres han reportado algunas inconsistencias con respecto a las diferencias en las regiones. Una variable confusora al comparar hombres y mujeres puede ser las diferencias en sus antecedentes genéticos y familiares. Un diseño que aborda dicha variable compara las estructuras cerebrales entre hermanos y hermanas, quienes comparten sus antecedentes genéticos y familiares.
En el presente estudio, apuntamos a contribuir a la literatura existente acerca de las diferencias sexuales cerebrales estructurales mediante la comparación del volumen por regiones de materia gris y blanca, usando Morfometría Basada en Vóxel (VBM), y la microestructura de la materia blanca usando Tract-based Spatial Statistics (TBSS) entre 40 hombres y mujeres sin relación familiar y contrastando dichos resultados con los obtenidos en un grupo de 47 hermanos de sexo opuesto, incluyendo 42 parejas de hermanos gemelos dicigóticos de sexos opuestos (DOS).
Nuestros resultados mostraron que los hombres tenían mayor volumen cerebral global, así como anisotropía fraccional de todo el cerebro por encima de la media, y también mostraron un volumen regional de materia gris incrementado y anisotropía fraccional más alta en, o alrededor, de las estructuras subcorticales (hipotálamo, tálamo, putamen y globo pálido, y el mesencéfalo anterior). En mujeres, el mayor volumen de materia gris se limitó a las áreas de la corteza, incluyendo las regiones temporal inferior, insular, cingulada, precentral y frontal/prefrontal.
Estas diferencias de sexo fueron generalmente consistentes entre las parejas de hombre-mujer sin relación familiar y las parejas de hermanos de sexos opuestos. Es por esto que concluimos que estas diferencias sexuales no son el resultado de diferencias confusoras en los antecedentes familiares o genéticos, y que la etiología de estas diferencias sexuales amerita más investigación.
Palabras Clave: Diferencias de sexo, gemelos de distintos sexos, imagen por resonancia magnética estructural, morfometría basada en Vóxel, imagen con tensor de difusión, anisotropía fraccional.
1. INTRODUCTION
Sex differences in human brain anatomy are thought to play a crucial role in the differential sensitivity to psychiatric disorders between males and females (Abel et al., 2010; Bekker and van Mens-Verhulst, 2007; Parker and Brotchie, 2010; Rucklidge, 2010) as well as in sex differences in specific cognitive abilities (Burgaleta et al., 2012; Halpern, 1997; Loring-Meier and Halpern, 1999; Mann, Sasanuma, Sakuma, and Masaki, 1990). The brains of males and females begin to differ in an early developmental stage through the action of sex specific factors, including hormonal, genetic and epigenetic factors (McCarthy and Arnold 2011), and sex-specific maturation continues during puberty and adolescence (Sisk and Zehr 2005). Post-mortem and in vivo imaging studies of both children and adults consistently report that males have an approximately 9-12% larger brain volume; and in addition, regional sexual dimorphisms have been found, primarily for areas with high numbers of sex steroid receptors. After correcting for total brain volume, morphological sex differences have been reported most frequently for basal ganglia and limbic structures, which include larger gray matter volumes in males for the amygdala and hypothalamus, and larger volumes in females for orbitofrontal cortex, hippocampus and caudate, for reviews see: (Cosgrove, Mazure and Staley, 2007; Giedd, Raznahan, Mills, and Lenroot, 2012; Lenroot and Giedd, 2010). However, previous findings have not always been consistent. For example, no difference in hippocampal and amygdalar volumes between males and females (Gur, Gunning-Dixon, Bilker, and Gur, 2002) or larger hippocampal volumes in males were also reported (Good et al., 2001).
For white matter, regions reported to show significant sex differences in volume, including the anterior temporal lobe and the internal capsule, which were found larger in males, and the posterior frontal lobe and optic radiation, found larger in females (Good et al., 2001). The corpus callosum has been found larger in females (Lacoste-Utamsing and Holloway, 1982), larger in males (Sullivan, Rosenbloom, Desmond, and Pfefferbaum, 2001), or of similar size in males and females (Bishop and Wahlsten 1997). In addition to regional volume, sex differences in white matter microstructure have been studied using diffusion tensor imaging (DTI), which measures the diffusion of water molecules in brain tissue. If there is no spatial structure, water molecules can move freely in all directions, which is referred to as isotropic diffusion. However, in WM tissue of the brain there is a directional selectivity due to the fact that water molecules diffuse more freely along neural axons than in any other direction, yielding anisotropic diffusion. One metric derived from DTI is the fractional anisotropy (FA), which is a scalar measure of the directional selectivity (Basser and Pierpaoli, 1996; Beaulieu, 2002; Le Bihan et al., 2001; Mori and Zhang, 2006). FA, which can vary from 0 (isotropic diffusion) to 1 (diffusion restricted to one axis), is thought to depend on the structure and density of neural axons and myelin sheaths and has been shown to decrease in several neurological and neuropsychiatric disorders (Assaf and Pasternak 2008).
Global FA averaged across the brain is generally larger in males (Abe et al., 2010; Inano, Takao, Hayashi, Abe, & Ohtomo, 2011; Kang, Herron and Woods, 2011) and, in line with this, higher FA values have been reported for many brain regions, including the thalamus, cingulate, occipito-parietal, temporal and frontal regions (Chou, Cheng, Chen, Lin, & Chu, 2011; Menzler et al., 2011; Schmithorst, Holland, & Dardzinski, 2008), as well as the corpus callosum (Menzler et al., 2011; Shin et al., 2005). However, regionally increased FA in females has also been found as for example in the fronto-occipital fasciculus and parahippocampal regions (Chou et al., 2011), left frontal lobe (Szeszko et al., 2003), and corpus callosum (Chou et al.; Schmithorst et al., 2008).
In summary, in addition to the general finding of larger global brain measures in males, studies point to regional sex differences primarily for basal ganglia and limbic brain structures. However, for regional differences there are also some inconsistencies that could have originated from many sources, including statistical power due to differences in sample size between studies, age of the subjects (e.g., adolescents versus adults), the analysis technique used (e.g. voxel-by-voxel comparison of fractional anisotropy versus tract-based spatial statistics [TBSS]), or whether or not differences in total brain size were accounted for (Giedd et al., 2012). An additional confound may be differences in family and genetic background, given that associations with global and regional brain structure have been reported for early developmental family environment (Buss et al., 2007; Rao et al., 2010; Yeo et al., 2013) and genetic makeup (den Braber et al., 2013; Thompson et al., 2001; Toga and Thompson 2005).
In the present study we aimed to contribute to the existing literature on sex differences in brain structure by comparing regional gray and white matter volume, using voxel based morphometry (VBM); and white matter microstructure, using TBSS between 87 adult men and 87 women. The sample consisted of 40 pairs of males and females of similar age with no family relationship, and 47 pairs of opposite-sex siblings, 42 of which were dizygotic twin pairs. Given that gray and white matter form an integrated neural circuit and recent evidence of shared underlying biological mechanisms (Kochunov et al., 2011a; Kochunov et al., 2011b), we expected that regional sex differences of gray matter converge with sex differences in white matter. The sex differences should be confirmed in the subsample of male-female sibling pairs.
2. METHODS
2.1. Participants
Participants were recruited from a study that investigated environmental and genetic influences on obsessive compulsive (OC) symptoms (den Braber et al., 2010). We selected scans of 40 men and 40 women with no family relationship who were closely matched for age. We refer to this group as the unrelated male-female subsample. A group of 5 male-female sibling pairs and 42 dizygotic male-female twin pairs constitutes the related subsample. In total, 56 (out of 174) participants showed high, but subclinical, OC symptom scores. For this study males and females were matched based on their OC symptom scores (making 28 high-scoring MF pairs [out of 87 MF pairs]). Excluding these subjects from the analyses did not change the overall results. All participants were aged between 18 and 60 years (Table 1). The twin pairs are the most optimal match possible, not only for nearly exact age, but also for early intrauterine and developmental environment and genetic make-up. The siblings are also matched for genetic and familial background, but differ in age (maximum age difference 5 years) and possibly for intrauterine environment. Exclusion criteria were brain damage, neurological disease, and contraindications for MRI (e.g., pregnancy, ferromagnetic fragments, clips and devices in the body and claustrophobia.
2.2. Protocol
Participants were administered diagnostic interviews and questionnaires, including questions on demography, life-events, and neuropsychiatric illness as described elsewhere (den Braber et al. 2010). Educational attainment was assessed as the highest level of education of the participant, divided into 3 categories: 1) lower general and vocational education; 2) intermediate vocational and intermediate/higher general education; 3) higher vocational college and university. The ethical review board of the VU university medical center approved the study protocol. All participants provided written informed consent.
2.3. Image acquisition
The MRI session consisted of an anatomical scan of about 6 minutes and a DTI scan of approximately 3 minutes. During the scan sessions, the participants remained inside the scanner and were asked to minimize head movement during and between consecutive runs. To reduce motion artifacts, each participants’ head was immobilized using foam pads.
MRI was performed on a 3.0 T Intera MR system (Philips, Medical Systems, Best) with a standard SENSE receiver head coil. The anatomical scan consisted of 182 coronal slices with a 3D T1-weighted gradient-echo sequence (Philips: T1TFE protocol without SPIR; flip angle: 8º; Repetition Time, TR: 9.69 ms; Echo Time, TE: 4.60 ms; matrix: 256x256 pixels; voxel size: 1.00x1.00x1.20 mm Field of View (ap,fh,rl): 218.4x256.0x256.0 mm). Diffusion tensor images were obtained in 32 directions by using single-shot echoplanar acquisition (Philips DwiSE protocol: one b = 0 scan, Repetition Time, TR: 4863 ms; Echo Time, TE: 94 ms; matrix: 112x110 pixels; voxel size: 2.0x2.0x3.00 mm; b-value: 1000 s/mm2, 38 axial slices; Field of View(ap,fh,rl): 230.0x114.0x230.0 mm).
2.4. Data analysis
Regional gray matter and white matter volume differences between males and females were analyzed using VBM as implemented in SPM8 (Wellcome Department of Imaging Neuroscience, London, UK). Twere segmented into gray matter, white matter and cerebrospinal fluid (CSF), and normalized to a group template (i.e., a specific template created from the 174 subjects in this study) using the Diffeomorphic Anatomical Registration Through Exponential Lie algebra (DARTEL) algorithm, and subsequently warped from DARTEL space to the 1-weighted MR images standard Montreal Neurological Institute (MNI) brain. To preserve volumetric information, a modulation step was added. Before statistical analysis, the resultant modulated images were spatially smoothed with an 8-mm isotropic Gaussian kernel.
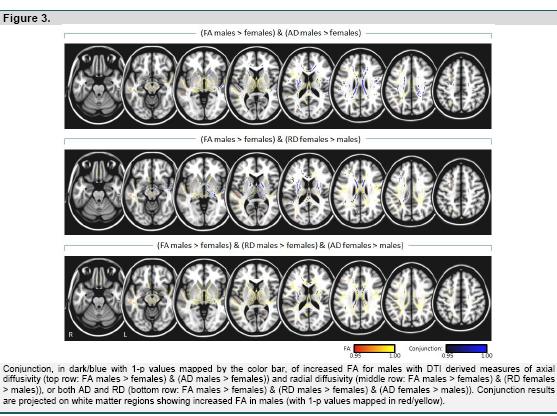
Sex differences in white matter diffusion were assessed using TBSS (Smith et al., 2006), part of FSL (Smith et al., 2004), which projects all subjects' fractional anisotropy data onto a mean fractional anisotropy tract skeleton, before applying voxel wise cross-subject statistics. This gives the opportunity to visualize WM differences on a true anatomical tract basis. In addition, we assessed the conjunction of significant sex difference for FA, with statistically significant sex differences in DTI derived measures of Axial Diffusivity (AD) and Radial Diffusivity (RD).
2.5. Statistical tests
Sex- differences in demographic and global brain measures were tested using paired sample t-tests or Chi-square test (SPSS, Chicago, Illinois), including a between pairs factors to account for sex difference by subsample (Unrelated vs. Related) interactions. Statistical results were considered significant at p < 0.05, Bonferroni corrected.
Per voxel sex differences in GM and WM volume, and FA, also were assessed using paired sample t-tests with a factor for sex difference by subsample (Unrelated vs. Related) interactions. For the SPM-VBM analyses of GM and WM, we corrected for differences in head size by including Total Intracranial Volume, computed as the sum of global GM, WM and CSF volumes, as a covariate. In addition, for the DTI-TBSS analyses, we assessed conjunctions of significant sex difference in FA values, with significant sex differences in axial diffusivity (AD) and/or radial diffusivity (RD), using the logical AND operation. That is, we looked for locations with individual sex difference test results of p < 0.05 for FA AND AD, for FA AND RD, and for FA AND AD AND RD; with the highest individual p-value taken as the conjunction significance. Finally, we also tested for significant correlations of sex differences with age.
For the SPM-VBM analyses on gray and white matter volume, statistical test results were considered significant at an individual voxel threshold of p < 0.05, FDR corrected. For the FSL-TBSS analyses on FA we applied a cluster based threshold of p < 0.05 with correction for multiple comparisons using Threshold-Free Cluster Enhancement in FSL (FSL randomise function with 10000 iterations).
3. RESULTS
3.1. Sample characteristics
Table 1 summarizes the characteristics of our sample, separately for the subsamples of unrelated male female pairs and opposite sex siblings. Mean age and education of the subjects in both subsamples were comparable (column Unrelated vs. Related). In addition, as expected, results within pairs tests for sex differences listed in table 1 indicate that there was no significant difference in age between the males and females in either subsample. There was also no significant sex difference for educational attainment in either subsample and no sex difference by subsample interaction (column Sex by Unrelated vs. Related).
3.2. Global brain measures
All global brain measures (TIV, GM, WM, FA) were larger in males than in females. Means, standard deviations and t-statistics for global brain measures are presented in the bottom rows of table 1. There were no differences between both subsamples in the absolute values, across males and females, of total intracranial volume (TIV), gray matter volume (GM), white matter volume (WM) or mean fractional anisotropy (FA) (column Unrelated vs. Related), as well as no sex difference by subsample interactions (column Sex x Unrelated vs. Related). The sex differences were also not influenced by age (Pearson correlation with age across all 87 pairs; TIV: r = .098, p=.368; GM: r = .084, p=.441; WM: r = .089, p=.414; mean FA: r = .158, p=.145).
3.3. Gray matter
Regional volumes (VBM: adjusted for total intracranial volume and age)
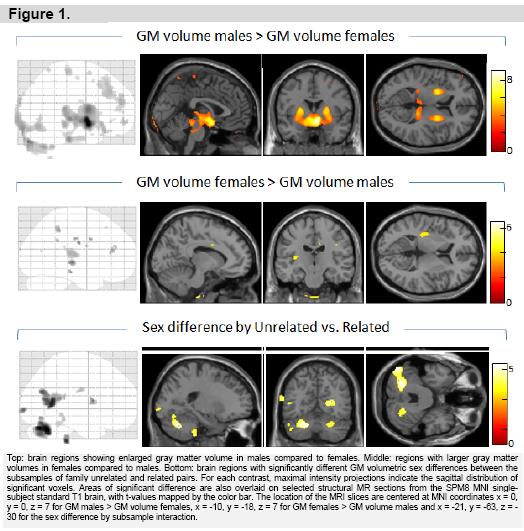
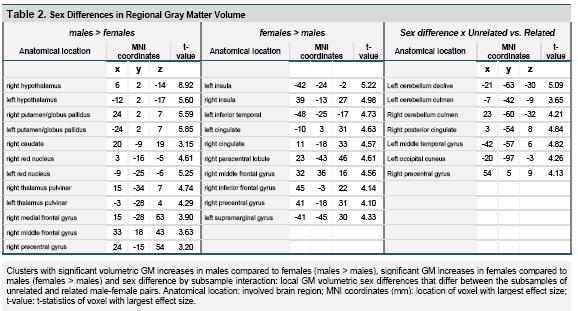
Figure 1 (top row) and table 2 (left) summarize GM regions found larger in males compared to females, across all 87 pairs. Significant enlargement of subcortical regions in males was seen in the hypothalamus, thalamus pulvinar, putamen, globus pallidus and right caudate, as well as the rostral midbrain. Clusters indicating relatively increased GM volume of the male cortex were found in the right middle and medial frontal gyri, and right precentral gyrus.
Analysis of GM regions with larger volume in females (figure 1, middle and table 2, middle) indicated a number of small clusters limited to the cortex. These included the left and right insula, left inferior temporal cortex, left and right cingulate, and a number of parietal and frontal lobe regions, mainly of the right hemisphere.
Assessment of sex difference by subsample interactions yielded a few significant clusters primarily in the posterior brain (figure 1, top and table 2, left). Post-hoc analyses indicated for most of these clusters (left cerebellum vermis declive, right cerebellum vermis culmen, left middle temporal gyrus, and left occipital cuneus) that the interaction was explained by volume enlargement in females compared to males of the unrelated pairs while a sex difference in the better matched related pairs was absent. For the right precentral region there was also enlargement for females in the unrelated pairs, but here with an opposite pattern of enlargement in males compared to females of the related pairs. Finally, for the right posterior cingulate and left cerebellum culmen, there was primarily volume enlargement in males of the related pairs. It should be noted however that none of these individual sex effects explaining the interactions for these regions, as described, showed up as statistically significant when testing for sex differences in the subsamples of related and unrelated male-female pairs, separately.
There were no significant effects of age on any of the observed sex differences in GM volume.
3.4. White matter
Regional volumes (VBM: adjusted for total intracranial volume and age)
The male-female comparison for regional white matter volume revealed no significant sex differences.
DTI- fractional anisotropy (TBSS: adjusted for age)
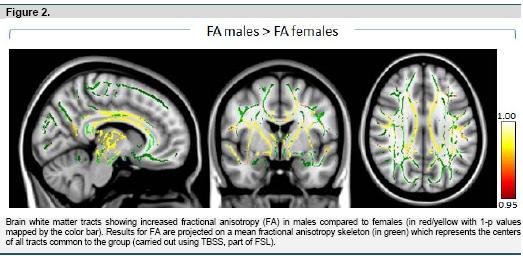
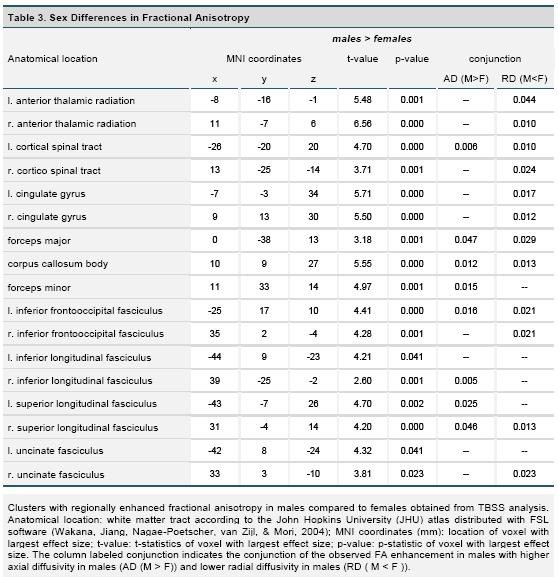
In the assessment of sex differences in fractional anisotropy (FA), we only observed clusters of significant differences using the contrast testing for increased FA in males. The findings are summarized in Figure 2 and table 3. Enhanced FA for males was present throughout the brain, including all major white matter fiber tracts. Tests for sex difference by subsample (Unrelated vs. Related) interactions for FA revealed no significant results.
For the complete sample we additionally investigated the conjunction of increased FA for males with DTI derived measures of axial diffusivity (AD) and radial diffusivity (RD). Results across the brain are shown in figure 3, and at locations with maximal statistics for the observed FA sex differences in table 3 (column labeled conjunction). Increased FA for males was accompanied throughout the brain by either significantly increased AD for males (figure 3: top row and table 3: column AD (M>F); e.g., regions of the inferior and superior longitudinal fasciculi), or decreased RD for males (figure 3: middle and table 3: column RD (M<F); e.g. within the thalamus), or both increased AD and decreased RD (figure 3: bottom row; e.g., body of corpus callosum).
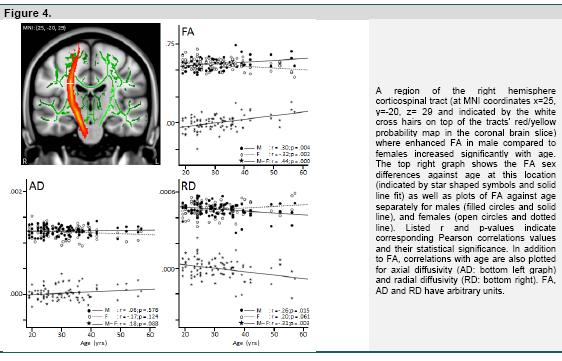
Assessment of the influence of age of the male-female pairs on the observed sex differences for FA, across all pairs, indicated a single significant cluster in a region of the right hemisphere corticospinal tract (figure 4: top left panel). Post-hoc analysis revealed that in this region there was a significant positive correlation of the males-females difference in FA with age, which could be explained by a significant increase with age of the FA values in males, and a significant decrease with age of the FA values in females (figure 4: top right panel). The pattern for axial diffusivity (AD) at this location was similar, but here correlations with age were not significant (figure 4: bottom left). For radial diffusivity (RD) the pattern was inverted, showing a significant negative correlation of the males - females difference in RD with age, explained by a significant decrease with age of the RD values in males, and a, nearly, significant increase with age of the RD values in females (figure 4: bottom right).
4. DISCUSSION
The present study aimed to create a comprehensive and consistent picture of sex differences in structural brain measures by comparing regional gray and white matter volume, and DTI of water diffusion between 40 males and females with no familial relationship, and between 47 sibling pairs, 42 of which were opposite-sex twins.
4.1. Sex differences in global brain measures
Males exhibited significantly larger total intracranial, gray and white matter volumes as well as higher mean fractional anisotropy across the brain. The finding of larger global brain volumes in males is in agreement with the previous literature and one of the most robust findings on sex differences in the brain, for review see: (Cosgrove et al., 2007; Lenroot and Giedd, 2010). Higher mean fractional anisotropy in males has also been found (Abe et al., 2010; Inano et al., 2011; Kang et al., 2011), which is consistent with the presumption of increased proportions of myelinated fibers for larger brains (Wang 2008). It has been reported that male brains possess higher neuronal densities, a higher number of neurons and fewer neuropil (i.e. neuronal and glial processes) (de Courten-Myers, 1999), with greater white matter volume available for inter-neuronal connections (Allen, Damasio, Grabowski, Bruss, & Zhang, 2003; Gur et al., 1999). From this, one might expect males to have fewer but thicker, more organized, and possibly more myelinated fibers.
4.2. Sex differences in regional gray matter
Males exhibited relatively increased gray matter volume primarily in subcortical brain regions, such as hypothalamus, thalamus, putamen and globus pallidus and rostral midbrain. Our finding of a larger hypothalamus is consistent with previous studies (Bao and Swaab, 2010; Goldstein et al., 2001). This brain region contains significant populations of sex steroid receptors, plays a central role in the control of sexual and reproductive function, has been related to sexual orientation and plays a major role in sexual arousal and psychosexual identity (the personal self-representation of being a 'male' individual) (Brunetti et al., 2008; Swaab, Chung, Kruijver, Hofman, & Ishunina, 2002). Together with the caudate nucleus, the putamen is the main receptive component of the basal ganglia. Anatomically, it is connected to the frontal cortex through a series of basal ganglia-thalamocortical loops that all run via the globus pallidus (Alexander, DeLong, & Strick, 1986; Haber 2003). Together with the thalamus and supplementary motor areas the putamen and globus pallidus are principally involved in the so-called motor loop that plays an important role in the programming and control of movement. Larger volumes of these regions or higher neural densities in males have also been found by others (Giedd, Castellanos, Rajapakse, Vaituzis, and Rapoport, 1997; Peper et al., 2009a). Interestingly, abnormalities within these regions have been linked to neuropsychiatric disorders that show a higher prevalence in males, such as tic disorders and schizophrenia (Abel et al., 2010; Shenton, Dickey, Frumin., & McCarley, 2001; Singer and Minzer, 2003; Singer and Walkup, 1991).
Increased gray matter volume for females, on the other hand, was restricted to areas of the cortex, including inferior temporal, insular, cingulate, precentral and frontal/prefrontal regions. Sexual differentiation for cortical regions may result from the impact of sex hormones (Goldstein et al., 2001) as well as sex chromosomes (McCarthy and Arnold 2011), such as the influence of genes that escape from X inactivation (Lentini, Kasahara, Arver, & Savic, 2013). Volume enlargement in insular, cingulate, and prefrontal regions in females have been observed previously, especially in studies that focussed on cortical thickness (Goldstein et al., 2001; Good et al., 2001; Im et al., 2006; Luders et al., 2006; Lv et al., 2010; Sowell et al., 2007). These structures play a major role in emotion and interoceptive awareness (the sense of the physiological condition of the body). The insula has been associated with both detection and experiencing disgust (Wicker et al., 2003), and also with interoceptive attention (Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004), while the anterior cingulate and lateral prefrontal cortices have been related to the integration of interoceptive information (Critchley et al., 2004). Moreover, the perception of bodily state, and simultaneous activation of cingulate, insular and prefrontal regions, was proposed a crucial determinant for the processing and subjective experience of feelings (Pollatos, Gramann, & Schandry, 2007). Interestingly, abnormalities within these brain regions have been found in neuropsychiatric disorders with higher prevalence in females, such as depression and anxiety disorders (Altemus 2006; Martin, Ressler, Binder, & Nemeroff, 2009; Palazidou 2012) and especially anxiety is highly associated with altered bodily responses, including sweating, increased heart rate and blood pressure.
Our finding of larger regional volumes in brain structures that play a central role in the control of sexual and reproductive function in males and in regions involved in more social emotional skills in females fits an evolutionarily perspective (Eagly and Wood, 1999), although we cannot discriminate this effect from a lifetime of socialization on the brains of males versus females. Experience can alter the brain as much as the brain can regulate experience (Paus, 2013).
4.3. Sex differences in regional white matter
Our VBM analyses did not reveal any significant difference in regional white matter volume. However, males and females had significantly different DTI derived fractional anisotropy, which consisted exclusively of increased FA in males. Increased FA was most significant within or close to the thalamus, and corpus callosum and cingulate bundles. Higher fractional anisotropy within and surrounding subcortical brain regions is in line with previous reports (Chou et al. 2011; Menzler et al. 2011) as is the higher fractional anisotropy in the corpus callosum and cingulum (Inano et al., 2011; Menzler et al., 2011; Shin et al., 2005), although findings for the corpus callosum have not been unequivocal (Chou et al., 2011; Schmithorst et al., 2008). Higher fractional anisotropy in other regions noted in our study, such as the frontal brain, is also consistent with the literature (Chou et al.; Schmithorst et al., 2008).
Conjunction analysis revealed different patterns of sex differences for DTI parameters within the brain. Within thalamic regions, increased FA in males was accompanied exclusively by reduced radial diffusivity indicating locally increased neuronal fiber myelination and myelin packing (Song et al., 2002; Song et al., 2005). In contrast, for the corpus callosum, there was a pattern of reduced radial as well as increased axial diffusivity which suggests both increased myelination and higher axonal quality (Song et al., 2003). In left and right cingulate areas increased FA was primarily paired with increased axial diffusivity only.
In general, we found no evidence for an increase or decrease in sex differences with increasing age. The exception was a region of the corticospinal tract, where we observed a sex by age interaction that was explained by a significant increase with age of FA in males, combined with a significant decrease with age of FA in females. The finding that these FA changes were accompanied by significant opposite age related trends for radial diffusivity (decrease of RD with age in males and increase of RD with age in females) suggest that the interaction is driven by an age related increase in myelination of the corticospinal tract for men and decreased myelination for women. Although several previous studies failed to find age-by-sex interactions for DTI indices (Hsu et al., 2008; Hsu et al., 2010; Inano et al., 2011), two recent studies did report significant results for a number of brain regions (Abe et al., 2010; Kochunov et al., 2012). In particular, Kochunov et al., (2012) reported age2 by sex interactions that also included the corticospinal tract, similar to our finding. Interestingly, Kochunov et al., furthermore found that out of 9 white matter tracts analysed, the corticospinal tract was the only exception in the sense that it did not show an age related trend for FA, across gender. This is consistent with the present finding of opposite age related trajectories of FA between males and females for this tract, that could cancel out age related changes. With respect to this, we should note however that, in contrast to the present findings, it has been more common to find a decrease in FA with age for both sexes, with age by sex effects due to a steeper decline with age in males (Abe et al. 2010; Kochunov et al. 2012). Kochunov et al. (2012) suggested that deviant age related findings for the corticospinal tract may be explained by the fact that the tract is unique in the sense that it is the earliest region to begin myelination during brain development, and is maintained by a special neurobiological process of myelination (i.e., by morphologically distinct oligodendrocytes that produce single myelin segments).
4.4. Unrelated male-female pairs versus opposite-sex siblings
Significant effects of matching for genetic background and shared family environment on sex differences were found only in our VBM gray matter analyses, and most prominently included left and right cerebellar regions. The interaction was explained by relative volume enlargement in females compared to males, which was restricted to the unrelated pairs. This suggests confounding by differences in early environmental and/or genetic background when comparing sex differences in unrelated males and females. The sex differences in these regions may therefore not hold up in replication. Indeed, in previous studies that reported sexual dimorphism for the cerebellum it has been more common to find enlarged volume in males, not females (Raz, Gunning-Dixon, Head, Williamson, & Acker, 2001; Rhyu et al., 1999; Tiemeier et al., 2010). In general, for both our volumetric and DTI diffusion analyses, the observed sex effects were consistent between family-unrelated and family-related males and females. This indicates that, at least at the sample size of 40 pairs, confounding by early environmental factors and genetic background in the comparison of unrelated males and females did not play a role for the presently analyzed brain anatomical variables. Furthermore, as we used opposite sex twin pairs, these findings bear on the issue of intrauterine steroid exposure. It has been hypothesized that females with a male co-twin might be exposed to higher testosterone levels and experience a relative masculinization of the brain. Evidence for a larger total brain volume in opposite-sex female twins compared to same-sex female twins has been reported for 9-year old children, but not for adults (Peper et al., 2009b). Masculinization could potentially make comparisons for DOS twin pairs more conservative, i.e. sex differences could be attenuated. The absence of the sample by sex interaction suggests that sex differences in the DOS twin sample are not significantly influenced by potential intrauterine testosterone exposure of the female twin.
In summary, this study shows males to have larger gray matter volumes in, or surrounding, subcortical structures. Consistent with this, increased DTI-FA in males was also most significant primarily for these regions. Increased FA in males at these locations showed conjunction with locally reduced radial diffusivity indicative of locally increased myelination. In contrast, females were characterized by larger gray matter volumes in cortical brain regions. These sex differences were consistent between the subsamples of unrelated male-female pairs and opposite-sex siblings and twins, indicating limited confounding by differences in genetic, and familial environmental factors or intrauterine testosterone exposure.
Assessment of sex differences in regional brain structure provide a rich source of information for understanding the behavioral differences between males and females in behavioral and other domains and should be considered in studies on the neurobiology of neuropsychiatric disorders that differ in prevalence or symptoms between the sexes.
5. FUNDING
This work was supported by the Netherlands Organisation for Scientific Research (MW 904-61-193, MaGW-nr: 400-07-080, MagW 480-04-004), and the Neuroscience Campus Amsterdam (AC-2009-F2-3; European Research Council (ERC-230374).
6. FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or other potential conflicts of interest.
7. ACKNOWLEDGMENTS
We thank Gabriëlla Blokland, Myrle Kemperman, Judith Wagner, Mira Geirnaert, Kim Meijer and Daisy van Minde for help with MRI data collection.
8. REFERENCES
Abe, O., Yamasue, H., Yamada, H., Masutani, Y., Kabasawa, H., Sasaki, H., ... Ohtomo, K. (2010). Sex dimorphism in gray/white matter volume and diffusion tensor during normal aging. NMR in Biomedicine, 23, 446-458. [ Links ]
Abel, K. M., Drake, R., Goldstein, J. M. ( 2010). Sex differences in schizophrenia. International Review of Psychiatry, 22, 417-428. [ Links ]
Alexander, G. E., DeLong, M. R., & Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9, 357-381. [ Links ]
Allen, J. S., Damasio, H., Grabowski, T. J., Bruss, J., & Zhang, W. (2003). Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage, 18, 880-894. [ Links ]
Altemus, M. (2006). Sex differences in depression and anxiety disorders: potential biological determinants. Hormones and Behavior, 50, 534-538. [ Links ]
Assaf, Y., & Pasternak, O. (2008). Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. Journal of Molecular Neuroscience, 34, 51-61. [ Links ]
Bao A. M., & Swaab, D. F. (2010). Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist, 16, 550-565. [ Links ]
Basser, P. J., & Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance - Series B, 111(3), 209-219. [ Links ]
Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine, 15, 435-455. [ Links ]
Bekker, M. H., van Mens-Verhulst, J. (2007). Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gender Medicine, 4(Suppl. B), S178-S193. [ Links ]
Bishop, K. M., & Wahlsten, D. (1997). Sex differences in the human corpus callosum: myth or reality? Neuroscience and Biobehavioral Reviews, 21, 581-601. [ Links ]
Brunetti, M., Babiloni, C., Ferretti, A., Del, G. C., Merla, A., Olivetti, B. M., & Romani, G. L. (2008). Hypothalamus, sexual arousal and psychosexual identity in human males: a functional magnetic resonance imaging study. European Journal of Neuroscience, 27, 2922-2927. [ Links ]
Burgaleta, M., Head, K., Alvarez-Linera, J., Martinez, K., Escorial, S., Haier, R., and Colom, R. (2012). Sex differences in brain volume are related to specific skills, not to general intelligence. Intelligence, 40(1), 60-68. [ Links ]
Buss, C., Lord, C., Wadiwalla, M., Hellhammer, D. H., Lupien, S. J., Meaney, M. J., & Pruessner, J. C. (2007). Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. The Journal of Neuroscience, 27, 2592-2595. [ Links ]
Chou, K. H., Cheng, Y., Chen, I. Y., Lin, C. P., & Chu, W. C. (2011). Sex-linked white matter microstructure of the social and analytic brain. Neuroimage, 54, 725-733. [ Links ]
Cosgrove, K. P., Mazure, C. M., Staley, J. K. (2007). Evolving knowledge of sex differences in brain structure, function, and chemistry. Biological Psychiatry, 62, 847-855. [ Links ]
Critchley, H. D., Wiens, S., Rotshtein, P., Ohman, A., & Dolan, R. J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7, 189-195. [ Links ]
de Courten-Myers, G. M. (1999). The human cerebral cortex: gender differences in structure and function. Journal of Neuropathology and Experimental Neurology, 58, 217-226. [ Links ]
den Braber, A., Bohlken, M. M., Brouwer, R. M., van 't Ent, D., Kanai, R., Kahn, R. S., ... Boomsma, D. I. (2013). Heritability of subcortical brain measures: A perspective for future genome-wide association studies. Neuroimage, 83, 98-102. [ Links ]
den Braber A, van 't Ent, D., Cath, D. C., Wagner, J., Boomsma, D. I., & de Geus, E. J. (2010). Brain activation during cognitive planning in twins discordant or concordant for obsessive-compulsive symptoms. Brain, 133, 3123-3140. [ Links ]
Eagly, A. H., & Wood, W. (1999). The origins of sex differences in human behavior - Evolved dispositions versus social roles. American Psychologist, 54, 408-423. [ Links ]
Giedd, J. N., Castellanos, F. X., Rajapakse, J. C., Vaituzis, A. C., Rapoport, J. L. (1997). Sexual dimorphism of the developing human brain. Progress in Neuro-psychopharmacology and Biological Psychiatry, 21,1185-1201. [ Links ]
Giedd J. N., Raznahan, A., Mills, K. L., Lenroot, R. K. (2012). Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biology of Sex Differences, 3,1-9. [ Links ]
Goldstein, J, M., Seidman, L. J., Horton, N. J., Makris, N., Kennedy, D. N., Caviness, V. S, Jr., ... Tsuang, M. T. (2001). Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex, 11, 490-497. [ Links ]
Good, C. D., Johnsrude, I., Ashburner, J., Henson, R. N., Friston, K. J., Frackowiak, R. S. (2001). Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage, 14, 685-700. [ Links ]
Gur, R. C., Gunning-Dixon, F., Bilker, W. B., & Gur, R. E. (2002). Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cerebral Cortex, 12, 998-1003. [ Links ]
Gur, R. C., Turetsky, B. I., Matsui, M., Yan, M., Bilker, W., Hughett, P., & Gur, R. E. (1999). Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. The Journal of Neuroscience, 19, 4065-4072. [ Links ]
Haber, S. N. (2003). The primate basal ganglia: parallel and integrative networks. Journal of Chemical Neuroanatomy, 26, 317-330. [ Links ]
Halpern, D. F., (1997). Sex differences in intelligence. Implications for education. American Psychologist, 52, 1091-1102. [ Links ]
Hsu, J. L., Leemans, A., Bai, C. H., Lee, C. H., Tsai, Y. F., Chiu, H. C., & Chen, W. H. (2008). Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage, 39, 566-577. [ Links ]
Hsu, J. L., Van, H. W., Bai, C. H., Lee, C. H., Tsai, Y. F., Chiu, H. C., ... Leemans, A. (2010). Microstructural white matter changes in normal aging: a diffusion tensor imaging study with higher-order polynomial regression models. Neuroimage, 49, 32-43. [ Links ]
Im, K., Lee, J. M., Lee, J., Shin, Y. W., Kim, I. Y., Kwon, J. S., & Kim, S. I. (2006). Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage, 31, 31-38. [ Links ]
Inano, S., Takao, H., Hayashi, N., Abe, O., & Ohtomo, K. (2011). Effects of age and gender on white matter integrity. American Journal of Neuroradiology, 32, 2103-2109. [ Links ]
Kang, X., Herron, T. J., Woods, D. L. (2011). Regional variation, hemispheric asymmetries and gender differences in pericortical white matter. Neuroimage, 56, 2011-2023. [ Links ]
Kochunov, P., Glahn, D. C., Lancaster, J., Thompson, P. M., Kochunov, V., Rogers, B., ...Williamson, D. E. (2011a). Fractional anisotropy of cerebral white matter and thickness of cortical gray matter across the lifespan. Neuroimage, 58,:41-49. [ Links ]
Kochunov, P., Glahn, D. C., Nichols, T. E., Winkler, A. M., Hong, E. L., Holcomb, H. H., ... Blangero, J. (2011b). Genetic analysis of cortical thickness and fractional anisotropy of water diffusion in the brain. Frontiers in Neuroscience, 5, 120. [ Links ]
Kochunov, P., Williamson, D. E., Lancaster, J., Fox, P., Cornell, J., Blangero, J., & Glahn, D. C. (2012). Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiology of Aging, 33, 9-20. [ Links ]
Lacoste-Utamsing, C., & Holloway R. L. (1982). Sexual dimorphism in the human corpus callosum. Science 216(4553), 1431-1432. [ Links ]
Le Bihan, D., Mangin, J. F., Poupon, C., Clark, C. A., Pappata, S., Molko, N., & Chabriat, H. (2001). Diffusion tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging, 13, 534-546. [ Links ]
Lenroot, R. K., & Giedd, J. N. (2010). Sex differences in the adolescent brain. Brain and Cognition, 72, 46-55. [ Links ]
Lentini, E., Kasahara, M., Arver, S., & Savic, I. (2013). Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cerebral Cortex, 23, 2322-2336. [ Links ]
Loring-Meier, S., Halpern, D. F., (1999). Sex differences in visuospatial working memory: components of cognitive processing. Psychonomic Bulletin and Review, 6, 464-471. [ Links ]
Luders, E., Narr, K. L., Thompson, P. M., Rex, D. E., Woods, R. P., Deluca, H., ... Toga, A. W. (2006). Gender effects on cortical thickness and the influence of scaling. Human Brain Mapping, 27, 314-324. [ Links ]
Lv, B., Li, J., He, H., Li, M., Zhao, M., Ai, L., ... Wang, Z. (2010). Gender consistency and difference in healthy adults revealed by cortical thickness. Neuroimage, 53, 373-382. [ Links ]
Mann, V. A., Sasanuma, S., Sakuma, N., Masaki, S., (1990). Sex differences in cognitive abilities: a cross-cultural perspective. Neuropsychologia, 28, 1063-1077. [ Links ]
Martin, E. I., Ressler, K. J., Binder, E., & Nemeroff, C. B. (2009). The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Psychiatric Clinics of North America, 32, 549-575. [ Links ]
McCarthy, M. M., & Arnold, A. P. (2011). Reframing sexual differentiation of the brain. Nature Neuroscience, 14, 677-683. [ Links ]
Menzler, K., Belke, M., Wehrmann, E., Krakow, K., Lengler, U., Jansen, A., ... Knake, S. (2011). Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage, 54, 2557-2562. [ Links ]
Mori, S., & Zhang, J. (2006). Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron, 51, 527-539. [ Links ]
Palazidou, E. (2012). The neurobiology of depression. British Medical Bulletin, 101, 127-145. [ Links ]
Parker, G., Brotchie, H. (2010). Gender differences in depression. International Review of Psychiatry, 22, 429-436. [ Links ]
Paus, T. (2013). How environment and genes shape the adolescent brain. Hormones and Behavior, 64, 195-202. [ Links ]
Peper, J. S., Brouwer, R. M., Schnack, H. G., van Baal, G. C., van Leeuwen, M., van den Berg, ... Hulshoff Pol, H. E. (2009a). Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology, 34, 332-342. [ Links ]
Peper, J. S., Brouwer, R. M., van Baal, G. C., Schnack, H. G., van Leeuwen, M., Boomsma, D. I., ... Hulshoff Pol, H. E. (2009b). Does having a twin brother make for a bigger brain? European Journal of Endocrinology, 160, 739-746. [ Links ]
Pollatos, O., Gramann, K., & Schandry, R. (2007). Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping, 28, 9-18. [ Links ]
Rao, H., Betancourt, L., Giannetta, J. M., Brodsky, N. L., Korczykowski, M., Avants, B. B., ... Farah, M. J. (2010). Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. Neuroimage, 49, 1144-1150. [ Links ]
Raz, N., Gunning-Dixon, F., Head, D., Williamson, A., & Acker, J. D. (2001). Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. American Journal of Neuroradiology, 22, 1161-1167. [ Links ]
Rhyu, I. J., Cho, T. H., Lee, N. J., Uhm, C. S., Kim, H., & Suh, Y. S. (1999). Magnetic resonance image-based cerebellar volumetry in healthy Korean adults. Neuroscience Letters, 270, 149-152. [ Links ]
Rucklidge, J. J. (2010). Gender differences in attention-deficit/hyperactivity disorder. Psychiatric Clinics of North America, 33, 357-373. [ Links ]
Schmithorst, V. J., Holland, S. K., & Dardzinski, B. J. (2008). Developmental differences in white matter architecture between boys and girls. Human Brain Mapping, 29, 696-710. [ Links ]
Shenton, M, E., Dickey, C. C., Frumin, M., & McCarley, R. W. (2001). A review of MRI findings in schizophrenia. Schizophrenia Research, 49, 1-52. [ Links ]
Shin, Y. W., Kim, D. J., Ha, T. H., Park, H. J., Moon, W. J., Chung, ... Kwon, J. S. (2005). Sex differences in the human corpus callosum: diffusion tensor imaging study. Neuroreport, 16, 795-798. [ Links ]
Singer, H. S., & Minzer, K. (2003). Neurobiology of Tourette's syndrome: concepts of neuroanatomic localization and neurochemical abnormalities. Brain and Development, 25(Suppl. 1), S70-S84. [ Links ]
Singer, H. S., & Walkup, J. T. (1991). Tourette syndrome and other tic disorders. Diagnosis, pathophysiology, and treatment. Medicine, 70(1), 15-32. [ Links ]
Sisk, C. L., & Zehr, J. L. (2005). Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology, 26,163-174. [ Links ]
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., ... Behrens, T. E. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage, 31, 1487-1505. [ Links ]
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen-Berg, ... Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23 (Suppl. 1), S208-S219. [ Links ]
Song, S. K., Sun, S. W., Ju, W. K., Lin, S. J., Cross, A. H., & Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage, 20, 1714-1722. [ Links ]
Song, S. K., Sun, S. W., Ramsbottom, M. J., Chang, C., Russell, J., & Cross, A. H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage, 17, 1429-1436. [ Links ]
Song, S. K., Yoshino, J., Le, T. Q., Lin, S. J., Sun, S. W., Cross, A. H., & Armstrong, R. C. (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage, 26, 132-140. [ Links ]
Sowell, E. R, Peterson, B. S., Kan, E., Woods, R. P., Yoshii, J., Bansal, R., ... Toga, A. W. (2007). Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebral Cortex, 17, 1550-1560. [ Links ]
Sullivan, E. V., Rosenbloom, M. J., Desmond, J. E., Pfefferbaum, A. (2001). Sex differences in corpus callosum size: relationship to age and intracranial size. Neurobiology of Aging, 22, 603-611. [ Links ]
Swaab, D. F., Chung, W. C., Kruijver, F. P., Hofman, M. A., & Ishunina, T. A. (2002). Sexual differentiation of the human hypothalamus. Advances in Experimental Medicine and Biology, 511, 75-100. [ Links ]
Szeszko, P. R., Vogel, J., Ashtari, M., Malhotra, A. K, Bates, J., Kane, J. M., ... Lim, K. (2003). Sex differences in frontal lobe white matter microstructure: a DTI study. Neuroreport, 14, 2469-2473. [ Links ]
Thompson, P. M., Cannon, T. D., Narr, K. L., van Erp, T. G., Poutanen, V. P., Huttunen, M., ... Toga, A. W. (2001). Genetic influences on brain structure. Nature Neuroscience, 4, 1253-1258. [ Links ]
Tiemeier, H., Lenroot, R. K., Greenstein, D. K., Tran, L., Pierson, R., & Giedd, J. N. (2010). Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage, 49, 63-70. [ Links ]
Toga, A. W., & Thompson, P. M. (2005). Genetics of brain structure and intelligence. Annual Review of Neuroscience, 28, 1-23. [ Links ]
Wakana, S., Jiang, H., Nagae-Poetscher, L. M., van Zijl, P. C., & Mori, S. (2004). Fiber tract-based atlas of human white matter anatomy. Radiology, 230, 77-87. [ Links ]
Wang, S. S. (2008). Functional tradeoffs in axonal scaling: implications for brain function. Brain, Behavior and Evolution, 72, 159-167. [ Links ]
Wicker, B., Keysers, C., Plailly, J., Royet, J. P., Gallese, V., & Rizzolatti, G. (2003). Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron, 40, 655-664. [ Links ]
Yeo, R. A., Martinez, D., Pommy, J., Ehrlich, S., Schulz, S. C., Ho, B. C., ... Calhoun, V. D. (2013). The impact of parent socio-economic status on executive functioning and cortical morphology in individuals with schizophrenia and healthy controls. Psychological Medicine, 1-9. [ Links ]