Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
International Journal of Psychological Research
Print version ISSN 2011-2084
int.j.psychol.res. vol.6 no.spe Medellín Oct. 2013
Self-rated Social-emotional Perception and Its Neurophysiologic and Cardiac Correlates While Viewing a Film Showing the Suffering of Other People
Percepción socio-emocional autoevaluada y sus correlativos neuropsicológicos y cardiacos al ver un film que muestra el sufrimiento de otras personas
Ilona Papouseka*, Elisabeth M. Weissa, Eva M. Reisera, Günter Schultera, H. Harald Freudenthalerb and Helmut K. Lacknera
a Department of Psychology, Biological Psychology Unit, University of Graz, Graz, Austria.
b Department of Psychology, Psychological Diagnostics Unit, University of Graz, Graz, Austria.
* *Corresponding author. Ilona Papousek, Karl-Franzens University of Graz, Department of Psychology, Univ.-Platz 2, A-8010 Graz, Austria. E-Mail: ilona.papousek@uni-graz.at.
Received: 06-08-2013-Revised: 14-09-2013-Accepted: 26-09-2013
ABSTRACT
Using electroencephalographic (EEG) and cardiac measures, the study examined relevant mechanisms that may explain individual differences in self-rated emotion perception (i.e., the propensity of perceiving the emotional states of other persons in everyday life). Healthy women (n = 122) were confronted with film scenes showing the suffering of other people. Functional coupling between prefrontal and posterior cortices, measured by EEG coherences, more strongly decreased in individuals higher on emotion perception. This finding suggests that the propensity to loosen prefrontal inhibitory control on posterior cortical areas involved in basic processes of emotion perception is associated with higher susceptibility to social-emotional information and, therefore, with higher scores on self-rated emotion perception. In addition, higher self-rated perception of other persons' emotions was related to more pronounced cardiac responses to the observation of horrifying events occurring to people in the film which indicate enhanced attention and heightened perceptual processing.
Key Words: emotion perception, EEG coherence, heart rate, cardiac defense response.
RESUMEN
Usando medidas electroencefalográficas (EEG) y cardiacas, el estudio examinó los mecanismos pertinentes que puedan explicar las diferencias individuales en la percepción de la emoción autoevaluada (es decir, la tendencia de percibir los estados emocionales de otras personas en la vida cotidiana). Las mujeres sanas (n = 122) fueron confrontadas con escenas de películas que muestran el sufrimiento de otras personas. El acoplamiento funcional entre la corteza prefrontal y posterior, medido por coherencias EEG, disminuyó más fuertemente en las personas con mayor percepción de la emoción. Este hallazgo sugiere que la propensión a relajar el control inhibitorio prefrontal en las áreas corticales posteriores implicados en los procesos básicos de la percepción de la emoción se asocia con una mayor susceptibilidad a la información socio-emocional y por tanto, con las puntuaciones más altas en la percepción de la emoción autoevaluada. Además, una mayor percepción autoevaluada de las emociones de otras personas estuvo relacionada con las respuestas cardíacas más explícitas a la observación de los terribles acontecimientos que ocurren a la gente en la película, lo cual indica una mayor atención e intensificación del procesamiento perceptual.
Palabras Clave: percepción de la emoción, coherencia EEG, frecuencia cardíaca, respuesta cardiaca de defensa.
1. INTRODUCTION
Emotion perception represents a fundamental aspect of social-emotional behaviour. It is essential for emotion communication, adaptive social behaviour, and normal social relationships (Bandura, 1986; Hobson, 1993; Izard, 2001). Several clinical conditions are associated with social disability as well as emotion perception difficulties, for instance, schizophrenia, autism, and depression (Harms, Martin, & Wallace, 2010; Kohler, Hoffman, Eastman, Healey, & Moberg, 2011; Kohler, Walker, Martin, Healey, & Moberg, 2010; Weiss et al., 2006). Explaining the neural mechanisms underlying individual differences in emotion perception and other social-emotional behaviours as well as their concomitants and consequences may broaden our understanding of the empirical basis of treatment, and may lead to an earlier diagnosis of atypical development that is not yet evident in behaviour and is not yet observable with behavioural methods (McPartland & Pelphrey, 2012).
Social-emotional perception as assessed in the current study refers to the self-rated susceptibility to social-emotional information in everyday life, that is, to the degree to which one tends to perceive the emotions of other people (Papousek, Freudenthaler, & Schulter, 2008, 2011; Papousek, Schulter, Lackner, Samson, & Freudenthaler, in press). In this approach, emotion perception is viewed as perceptual in a narrower sense and from a social-emotional perspective, referring to mostly automatic processes of perceiving the emotional state of other persons through observable information such as facial expression, vocal inflections, or some combination of these that do not require cognitive effort (Couture, Penn, & Roberts, 2006).
Brain processes explaining individual differences in emotion perception according to this definition very likely include mechanisms modulating the extent or depth to which social-emotional perceptual input is processed. Theory and research evidence suggest that attention to relevant stimuli plays a crucial role in social-emotional perception. Most relevant research referred to the perception of facial emotional expressions. For instance, it has been shown that emotion perception requires that individuals select which parts of the face to attend to and then maintain their attention to collect relevant information about another's emotional state (e.g., Addington & Addington, 1998; Combs & Gouvier, 2004). Moreover, current models of potential brain mechanisms involved in the processing of social-emotional information assume the contribution of both a bottom-up and a top-down component. The bottom-up process which is automatically activated by perceptual input is supposed to be modulated in a top-down fashion through an executive control component implemented in prefrontal cortex (see Decety & Moriguchi, 2007 for review). These modulatory processes are also automatic processes, activated without any deliberate effort, and determine how much impact the perceptual input has on the individual (see e.g., Papousek et al., 2013; Reiser et al., 2012). Basic processes required for emotion perception (mainly processing information about the changeable configuration of faces such as facial expressions, eye movements, mouth movements) are located in temporal and anterior parietal regions of the cortex, especially in and near the superior temporal gyrus (Haxby, Hoffman, & Gobbini, 2000; Puce, Allison, Bentin, Gore, & McCarthy, 1998). Accordingly, essential mechanisms modulating the processing of social-emotional perceptual input include functional communication between prefrontal and temporoparietal cortices. Note that, although terms are often used interchangeable, the concept of social-emotional perception used in the current study does not refer to primarily cognitive processes subsumed under the terms theory of mind or cognitive perspective-taking, which refer to inferences about thoughts, intentions, and beliefs of others, or to emotion recognition, which refers to the correct identification of emotions.
Increases of EEG (electroencephalogram) coherence are considered to indicate increased connectivity and functional communication between two neuronal populations (Fries, 2005; Srinivasan, Winter, Ding, & Nunez, 2007). Recent research suggested that state-dependent changes of EEG coherence between prefrontal and temporoparietal cortical regions are indicative of a mechanism modulating the impact social-emotional information has on the individual. Two studies indicated that diminished prefrontal-posterior coupling, indicated by reduced EEG coherences, was related to reduced inhibitory control of the prefrontal cortex over incoming social-emotional information and, consequently, to a greater impact of the perceptual input on the individual (Papousek et al., 2013; Reiser et al., 2012). Thus, less top-down inhibition of the temporoparietal areas involved in the basic processes of emotion perception by prefrontal cortex may make an individual more susceptible to other people's affective expressions and, therefore, may result in a greater propensity to perceive other persons' emotions.
As explained above, social-emotional perception as assessed in the current study mostly refers to perceptual processes. In line with this, the examination of EEG coherences in this context refers to the automatic modulation of perceptual input.
However, empirical evidence suggested that the used self-report emotion perception scale to some extent also relates to the emotional impact of the perceptual input, that is, to which extent individuals tend to be affected by the emotions of other persons (Papousek et al., 2008, 2011).
The emotional impact of perceived information is reflected in an individual's heart rate response. Compared to subjective ratings, physiological measures such as heart rate have the advantage that emotional responses can be assessed independently from cognitive evaluation (Baumgartner, Esslen, & Jänke, 2006), and thus, physiological measures are indicative of more automatic response tendencies. During a stressful film depicting the suffering of other people such as news coverage of disaster, rape, etc., heart rate typically increases depending on the felt distress (Weidmann Conradi, Gröger, Fehm, & Fydrich, 2009). Generally, the magnitude of heart rate increase during viewing emotion-eliciting films of several minutes is correlated with subjective ratings of arousal or intensity of felt emotions (Fernandez et al., 2012).
In addition, using higher time resolutions, that is, analyzing the time course of heart rate changes across a series of time frames of a few seconds allows the assessment of transient processes that may supply information not available from average measures across the entire stimulation period of several minutes (Lackner, Batzel, Rössler, Hinghofer-Szalkay, & Papousek, 2013). Specifically, transient responses to the observation of aversive social-emotional cues can give information on processes related to externally oriented attention and sensory intake. Acute cardiac responses to an intense, unexpected, and aversive stimulus are typically characterized by initial fast heart rate acceleration, deceleration, and again (more gradual) acceleration, constituting the so-called cardiac defense response (Graham & Clifton, 1966; Vila et al., 2007). The maximum deceleration occurs approximately 10 s after stimulus onset (e.g., Keil et al., 2010; Ramirez, Sanchez, Fernandez, Lipp, & Vila, 2005; Vila et al., 2007). It has been proposed that the cardiac "defense" response reflects heightened attention allocation and sensory intake (Bradley, 2009; Lang, Bradley, & Cuthbert, 1997), which has been corroborated by empirical evidence: Studies found a positive relationship between the magnitude of the cardiac "defense" response and external attention (for review see Vila et al., 2007). Strong cardiac "defense" responses were accompanied by enhanced cortical sensitivity to external visual cues which was not specific to threat cues, that is, they were accompanied by facilitated visual perception (Keil et al., 2010). When direction of attention was experimentally manipulated toward external (compared to internal) cues, there was a clear potentiation of the transient cardiac response (Perez, Fernandez, Vila, & Turpin, 2000; Vila, Perez, Fernandez, Pegalajar, & Sanchez, 1997). Thus, a greater cardiac response to the observation of horrifying events occurring to other people may reflect greater attention to and sensory intake of social-emotional information and, therefore, may be correlated with a greater propensity to perceive other persons' emotions in everyday life.
Taken together, the present study aimed at clarifying some of the mechanisms that may explain individual differences in the propensity of perceiving the emotional states of other persons in everyday life. By using several neurophysiological methods supposedly indicative of specific processes, we aimed at gaining a more complete picture of the factors explaining individual differences in self-reported social-emotional perception. Transient heart rate responses to marked terrifying events are indicative of individual differences related to externally oriented attention and sensory intake. By examining changes of prefrontal-posterior EEG coherences, the importance of processes related to the automatic modulation of perceptual input by prefrontal cortex was tested. The examination of the magnitude of heart rate increase during viewing the film refers to the extent to which the participants were emotionally affected by the perceptual input. Healthy participants were confronted with film scenes displaying horrifying events such as road traffic accidents and the suffering of dying, severely injured, and mourning people while their EEG and heart rate were recorded. This protocol was chosen because of its exceptional ecological validity. The processing of observing the suffering of other people in real life but also through televised images has also proven relevance to psychopathological developments (Breslau, Bohnert, & Koenen, 2010; Durham, McCammon, & Alison, 1985; Holmes, Brewin, & Hennessy, 2004; Schuster et al., 2001).
We expected that participants scoring higher on self-rated emotion perception in everyday life would show relatively greater decreases of functional coupling of prefrontal and temporoparietal cortices (EEG coherences) and might show higher average heart rate increases while viewing the film. In addition, we expected that the individuals' habitual emotion perception would moderate their transient heart rate responses to marked terrifying events, that is, that participants higher on self-rated emotion perception would show a more pronounced cardiac response to these events. To confirm that the effects of the participants' self-rated perception of other persons' emotions were specific, that is, that they were not due to generally self-confident ratings of their social-emotional behaviour or to emotion-related competency in general, self-estimates of other interpersonal emotional behaviour (regulation of other persons' emotions) and of intrapersonal emotional behaviour (perception and regulation of one's own emotions) were statistically controlled.
2. METHOD
2.1. Participants
The sample comprised 122 right-handed female university students aged between 18 and 38 years (M = 22.2, SD = 3.6). Only individuals who confirmed that they did not have traumatic experiences related to car crashes, surgery or death of a close person within the past twelve months and did not have a neuropsychiatric disease or using psychoactive medication were admitted to participate. The absence of severe depressive symptoms was confirmed using the Beck Depression Inventory (German adaptation, Hautzinger, Bailer, Worall, & Keller, 1991). A female only sample was chosen because previous research has suggested sex differences in emotion perception performance as well as in the neural pathways underlying the processing of other persons' emotions (Kessels, Montagne, Hendriks, Perrett, & de Haan, in press; Scholten, Aleman, Montagne, & Kahn, 2005; Weisenbach et al., in press). Moreover, there is evidence of multi-faceted sex differences in autonomic nervous system responsiveness to stress (Kajantie & Phillips, 2006), and previous research indicated that women are more reactive to negative emotional stimulation than men, particularly when the stimulation is threatening or traumatic (Whittle, Yücel, Yap, & Allen, 2011). Handedness was assessed by a standardised hand skill test (Hand Dominance Test; Papousek & Schulter, 1999; Steingrüber & Lienert, 1971). Participants were requested to refrain from alcohol for twelve hours and from coffee and other stimulating beverages for two hours, prior to their lab appointment, and to come to the session well rested. The study was performed in accordance with the American Psychological Association's Ethics Code and the 1964 Declaration of Helsinki and was approved by the local ethics committee. Participants gave their written and informed consent to participate in the study.
2.2. Social-emotional Stimulation
Participants were exposed to a film (approx. 10 min in length) containing 11 clips including graphic scenes of severely injured, dying, and mourning people. EEG and ECG (electrocardiogram) were recorded during the last five minutes of the film during which there were five clips depicting a rampaging elephant injuring people at a circus and several car accidents. The film clips were used in previous studies (Holmes & Bourne, 2008; Holmes, James, Coode-Bate, & Deeprose, 2009; Holmes, James, Kilford, & Deeprose, 2010). The film content was similar to that witnessed by television viewers watching programs such as news coverage of road traffic accidents, or reality programs about the police or ambulance service work. The short stories told by the film scenes contained marked terrifying events and segments with negative content depicting the suffering of people, as well as short segments with neutral or mildly positive content that preceded the terrifying events. The film was displayed on a 21" computer monitor viewed at 100 cm and was presented without sound, so that the stimulation was dominated by the visual information for all participants. The neutral visual display, used for obtaining the reference data, showed a green circle (diameter 75 mm) at the center of the screen. This display was used in previous studies on changes of EEG coherences in the context of affective processing (Papousek et al., 2013; Reiser et al., 2012).
2.3. Prefrontal-posterior EEG Coherences: Recording and Quantification
EEG was recorded from 19 channels according to the international 10-20 system, using a Brainvision BrainAmp Research Amplifier (Brain Products; sampling rate 500 Hz, resolution 0.1µV) and a stretchable electrode cap, referenced to the nose and re-referenced offline to a mathematically averaged ears reference (Essl & Rappelsberger, 1998; Hagemann, 2004). Impedance was kept below 5 kΩ for all electrodes. Horizontal and vertical EOG measures were obtained for identification of ocular artifacts. All data were inspected visually, in order to eliminate intervals in which ocular or muscle artefacts occurred. All participants had at least 30 s of artefact-free data in each of the recording periods and in each of the electrode positions of interest. Artefact-free EEG data were submitted to Fast Fourier Analysis using a Hanning window (epoch length 1 s, overlapping 50%, low-cut filter 0.016 Hz). Spectral coherence (Fisher's z-transformed) was obtained in the beta frequency range (13-30 Hz) using the quotient of the cross spectrum (CS) and the auto spectra (power spectra) according to the following equation: Coh(c1, c2)(f) = |CS(c1, c2)(f)|2 / (|CS(c1, c1)(f)| ⋅ |CS(c2, c2)(f)|), with CS(c1, c2)(f) = Σc1,i (f) c2,i (f). Coh(c1, c2)(f) denotes the coherence at frequency f between electrodes 1 and 2, which can vary between 0 and 1.
We focused on EEG coherence in the beta frequency range, because previous research on EEG coherence in the context of affective processing indicated that connectivity changes during evoked emotions occurred primarily in the beta frequency range (Aftanas, Lotova, Koshkarov, & Popov, 1998; Miskovic & Schmidt, 2010; Papousek et al., 2013; Reiser et al., 2012; Schellberg, Besthorn, Klos, & Gasser, 1990). Research also suggested a particular importance of beta-band oscillations for mediating long distance coupling (Gross et al., 2004; Kopell, Ermentrout, Whittington, & Traub, 2000; Schnitzler & Gross, 2005).
Following previous relevant research (Miskovic & Schmidt, 2010; Papousek et al., 2013; Reiser et al., 2012), coherence pairs were grouped into anatomically valid clusters corresponding to the left and right, prefrontal and posterior association cortex regions. Coherence scores of nine electrode pairs each were averaged to summarise interaction within the left and the right hemisphere, respectively (left: Fp1-T3, Fp1-P3, Fp1-T5, F3-T3, F3-P3, F3-T5, F7-T3, F7-P3, F7-T5; right: Fp2-T4, Fp2-P4, Fp2-T6, F4-T4, F4-P4, F4-T6, F8-T4, F8-P4, F8-T6). By using these clusters we avoided a hardly manageable inflation of the number of statistical tests. The selection of the posterior electrodes was in accordance with evidence of involvement of the posterior part of the temporal lobe and the inferior parietal lobe in the visual perception of socially relevant information (Decety & Sommerville, 2003; Haxby et al., 2000; Puce et al., 1998).
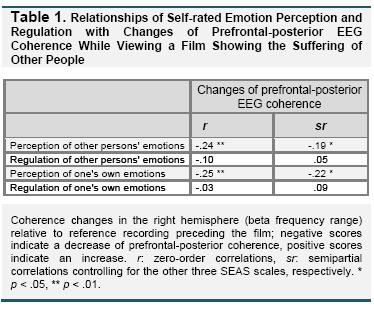
Following previous research on EEG coherence changes in affective contexts, linear regressions were conducted using the EEG beta coherence during the reference period preceding the film to predict the coherence during viewing the film, in order to calculate residualized change scores (Papousek et al., 2013; Reiser et al., 2012). These were used as an index of state-dependent decreases or increases of intrahemispheric coherence in response to watching other people suffer. This was done to ensure that the analysed residual variability was due to the experimental manipulation, and not to individual differences in baseline levels, and to control for measurement error inherent in the use of repeated measures of the same kind (e.g., Linden, Earle, Gerin, & Christenfeld, 1997; Steketee & Chambless, 1992). In the following, the abbreviation ‘‘Δcoh’’ will be used for these change-of-coherence scores. Negative scores indicate a decrease in prefrontal-posterior coherence, positive scores indicate an increase.
2.4. Cardiac Responses: Recording and Quantification
The electrocardiogram (ECG) was recorded using a Brainvision BrainAmp ExG Research Amplifier (Brain Products, sampling rate 500 Hz), using Ag-AgCl electrodes and a standard limb lead II electrode configuration. Interbeat intervals were derived using QRS complex detection based on Hilbert transformation (Harke, Schlögl, Anderer, & Pfurtscheller, 1999; Nygards & Sörnmo, 1983). Single artefacts were replaced by interpolation. On average M = 99.0 (SD = 1.5) and M = 99.6 (SD = 0.6) percent of QRS complexes were valid for the reference recording and the recording during the film, respectively. Interbeat intervals were used for calculating mean heart rates for the reference period preceding the film and the recording while viewing the film. For the analysis of transient heart rate responses, interbeat intervals were resampled at 4 Hz using piecewise cubic spline interpolation. The third-order polynomial trend of the individual heart rate time series was removed, and heart rate time series averaged across all participants were calculated to identify local heart rate extremes within the film scenes. Heart rate time series from 10 s preceding the most marked heart rate minima (indicating responses to the most marked horrifying events in the scenes) to 14 s after these minima were segmented in 4-s intervals, in order to reduce breathing frequency effects. This procedure supplies a segment of the cardiac "defense" response, timed by an objective criterion. (Extraction of a longer segment was not possible, because other events interfered). Values for these 4-s intervals were referenced to the reference period and were averaged across the three most marked horrifying events to obtain the transient heart rate responses (relative heart rate scores of six 4-s intervals) used in the statistical analysis. The three most marked horrifying events comprised of a boy playing in the garden killed by a car veering off the street, a teenager crossing the street while writing a SMS being run over by a car, and a couple in love sitting on a wall being hit by a car out of control. In addition, a 24-s time frame with neutral / mildly positive content was selected (relative scores of six 4-s intervals). This time frame comprised of a boy playing football in the garden and adult football players scoring a goal and celebrating in the pub.
2.5. Self-report Measures
The Self-report Emotional Ability Scale (SEAS; Freudenthaler & Neubauer, 2005; see also Papousek et al., 2008, 2011; Papousek, Schulter, et al., in press) is designed for assessing the self-reported handling of emotion in everyday life. It includes two subscales referring to interpersonal social-emotional behaviour and two subscales referring to intrapersonal emotional processes. The two scales concerning interpersonal social-emotional behaviour refer to "perception of other persons' emotions" (11 items, e.g., "I can tell immediately if a friend is worrying about something"; internal consistency reliability in the present sample is α = .81) and "regulation of other persons' emotions" (8 items, e.g., "I can influence the mood of others very well", α = .79). The two scales concerning intrapersonal emotion processing refer to "perception of one's own emotions" (9 items, e.g., "It often takes a long time of deliberation for me to realise that I'm envious of someone", α = .79), and "regulation of one's own emotions" (6 items, e.g., "When I'm scared of something I barely can't do anything about it", α = .75).
A rating scale assessed the subjective impact of the stimulation. After a two-minutes resting period following the film, participants retrospectively rated the degree to which the film had affected them (10 cm horizontal visual analogue scale scored in millimeters from 0 "not at all" to 100 "extremely"; M = 73.9, SD = 21.5).
2.6. Procedure
After completing the handedness test and some questionnaires that are not relevant to the present research question, participants were seated in an acoustically and electrically shielded examination room and electrodes were attached. Participants were instructed that after a short recording period during which they should watch the green circle on the screen (2 min) they would see a film to which they should direct their whole attention. They were asked to view the film as if they were really there, like a bystander at the scene of the events and to not close their eyes or look away. The film would be followed by another 2-min resting period. Subsequently, the participants completed the rating scales using the computer mouse. The experimenter was positioned outside the examination room, and participants were monitored using a camera. The SEAS was administered in a second, separate testing session one week later1.
2.7. Statistical Analysis
To evaluate whether the participants' self-rated emotion perception in everyday life may predict inter-individual differences in the EEG coherence changes during observing the suffering of other people, two multiple regression analyses were conducted, one using the change-of-coherence score (Δcoh) in the right hemisphere as the dependent variable and one using Δcoh in the left hemisphere as the dependent variable. The four SEAS scales were simultaneously entered as predictors to test for unique effects of the participants' self-rated perception of other persons' emotions, independently of the intrapersonal aspects of emotional behaviour and their rating of the ability to influence the moods of others. Coherence changes were analysed separately for the left and the right hemisphere, because previous research suggested lateralized effects of prefrontal-posterior coupling on affective processing (Papousek et al., 2013; Reiser et al., 2012; Schellberg et al., 1990).
The moderating effects of emotion perception on heart rate responses during viewing the film were examined by three one-way repeated-measures analyses of covariance (ANCOVAs) using the four SEAS scales as covariates (continuous between-subjects factors). One analysis examined the effect on transient heart rate responses using the relative scores of the six extracted 4-s intervals in the context of the most marked horrifying events as the levels of the within-subjects factor. In an analogous analysis, the relative scores of six 4-s intervals during a film period with neutral / mildly positive content were used as the levels of the within-subjects factor. The third analysis examined the effect on the average heart rate during the entire 5-min film period compared to the reference period preceding the film. Again, the four SEAS scales were simultaneously entered to test for unique effects. The multivariate approach to repeated measures analyses was used, which allows valid tests under nonsphericity conditions (Vasey & Thayer, 1987). A significant interaction between the within-subjects factor and the SEAS perception of other person's emotions score indicates a moderating effect of the individuals' habitual perception of other person's emotions on their heart rate responses during viewing the film, that is, that the heart rate changes depended on the individual's self-reported emotion perception in everyday life. To illustrate significant interaction effects, predicted heart rate values for each level of the within-subjects factor were calculated for one standard deviation below and one standard deviation above the mean on the SEAS scale, using multiple regression analysis with the four SEAS scales as predictors.
Effects on the subjective impact of the film were evaluated using multiple regression analysis with the four SEAS scales as predictors and the rating scale as the dependent variable. Interrelations among the physiological measures were tested by two Pearson correlations (Δcoh in the right / left hemisphere by global heart rate change film minus reference period), and the interaction in three oneway repeated-measures ANCOVAs using Δcoh in the right / left hemisphere or global heart rate change as the covariate to evaluate their potential relationships to the transient heart rate responses.
Standard software (SPSS 20) was used for all analyses.
3. RESULTS
3.1. Prefrontal-posterior EEG Coherences
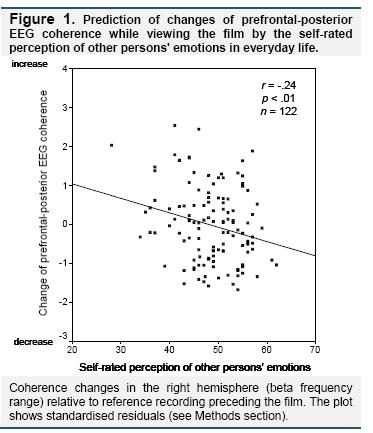
The regression analysis with Δcoh in the right hemisphere as the dependent variable showed that self-reported perception of other persons' emotions predicted to which degree EEG coherence increased or decreased during viewing the film (B = -0.03, SE = 0.02, β = -.22, p < .05; F(4,117) = 3.4, p < .05; R² = .11). Higher scores on the SEAS perception of other persons' emotions scale were associated with a stronger decrease of prefrontal-posterior coherence during viewing the film. Independently from that, higher scores on the SEAS perception of one's own emotions scale predicted greater decreases of Δcoh in the right hemisphere (B = -0.04, SE = 0.01, β = -.25, p < .05). No significant relationships were observed for regulation of other persons' emotions (B = 0.01, SE = 0.02, β = .06, ns.) and regulation of one's own emotions (B = 0.02, SE = 0.02, β = .10, ns.). Zero-order and semipartial correlations are shown in Table 1. Figure 1 shows the scatterplot of the correlation between the perception of other persons' emotions scale and EEG coherence changes during viewing the film.
In the regression analysis with Δcoh in the left hemisphere as the dependent variable, the total regression model was not significant (F(4,117) = 2.0, ns.)2.
3.2. Cardiac Responses
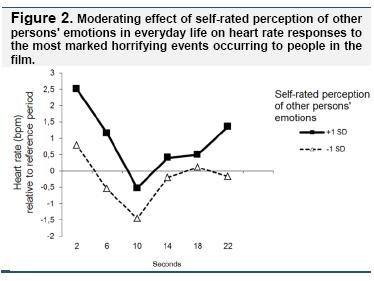
In the analysis examining the transient heart rate responses to the most marked horrifying events, the relevant interaction effect of time frame by perception of others' emotions was significant (F(5,113) = 2.6, p < .05, ηp2 =10). The effect is illustrated in Figure 2. It shows that the time course of heart rate changes in participants higher and lower on perception of others' emotions had a similar shape. However, participants higher on perception of others' emotions showed higher initial heart rate acceleration as well as stronger heart rate deceleration (note the steeper slope of the black line compared to the dotted line, with the difference between the lines at second 2 being greater than that at second 10). Moreover, the second acceleration was more pronounced in those higher on perception of other persons' emotions. Taken together, the transient heart rate responses indicated a more pronounced response to the most significant events of the film when participants scored higher on the perception of others' emotions scale. No other interaction effects were significant (regulation of other persons' emotions: F(5,113) = 2.3, ns.; perception of one's own emotions F(5,113)=1.6, ns.; regulation of one's own emotions F(5,113) = 1.1, ns.), and there were no significant main effects (time frame: F(5,113) = 1.0, ns.; perception of others' emotions: F(1,117) = 1.2, ns.; regulation of others' emotions: F(1,117) = 2.3, ns.; perception of one's own emotions: F(1,117) = 0.02, ns.; regulation of one's own emotions: F(1,117) = 0.01, ns.).
The analysis of the film period with neutral / mildly positive content showed no main effects (perception of others' emotions: F(1,117) = 0.8, ns.; regulation of others' emotions: F(1,117) = 0.8, ns.; perception of one's own emotions: F(1,117) = 0.03, ns.; regulation of one's own emotions: F(1,117) = 0.08, ns.; time frame: F(5,113) = 0.3, ns.) nor any significant interaction effects (perception of other persons' emotions: F(5,113) = 1.0, ns.; regulation of other persons' emotions: F(5,113) = 1.0, ns.; perception of one's own emotions F(5,113) = 0.7, ns.; regulation of one's own emotions F(5,113) = 0.3, ns.).
The analysis examining the effect of the participants' self-rated social-emotional behaviour on their global heart rate changes from the reference period preceding the film to viewing the film yielded no significant results (period: F(1,116) = 0.02, ns.; perception of others' emotions x period: F(1,116) = 0.04, ns.; regulation of others' emotions x period: F(1,116) = 0.9, ns.; perception of one's own emotions x period: F(1,116) = 0.4, ns.; regulation of one's own emotions x period: F(1,116) = 0.2, ns.).
3.3. Supplementary Analyses
The total regression model with the rating to which degree the film had affected the participants as the outcome variable failed to be significant (F(4,117) = 1.6, ns.).
The physiological measures were not substantially interrelated, confirming that they explain different portions of variance (correlation between Δcoh and global heart rate change right hemisphere r = -.08, ns., left hemisphere r = .05, ns.; interaction time frame of the transient heart rate response by Δcoh right hemisphere F(5,116) = 0.8, ns., left hemisphere F(5,116) = 1.4, ns.; interaction time frame of the transient heart rate response by global heart rate change F(5,116) = 1.1, ns.).
4. DISCUSSION
The present study investigated a neural mechanism potentially underlying individual differences in the perception of other persons' emotions, that is, changes in prefrontal-posterior coupling during confrontation with social-emotional information. To this end, EEG coherences were obtained while participants were viewing a film showing the suffering of other people. In addition, cardiac responses to the observation of horrifying events occurring to people in the film were analysed, in order to study whether these responses may also be moderated by individual differences in emotion perception.
Prefrontal-posterior EEG coherence more strongly decreased while viewing the film scenes in individuals higher on self-rated emotion perception. According to previous research this can be interpreted as less top-down inhibition exerted by prefrontal cortex on posterior cortical areas that are involved in basic processes of emotion perception (Papousek et al., 2013; Reiser et al., 2012). The finding suggests that the propensity to loosen prefrontal inhibitory control over posterior cortices in the presence of other people is associated with higher susceptibility to social-emotional information and, therefore, with higher emotion perception. This interpretation is in line with neuroscientific models on affective processing implicating pathways originating from prefrontal cortex that modulate the activity of other brain structures (Davidson, 2002; Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007; Phillips, Ladouceur, & Drevets, 2008). While most research has been concerned with pathways from the prefrontal cortex to the amygdala, it is being increasingly recognised that not only cortical-subcortical, but also cortico-cortical circuits play an important role in affective processing. Remote brain regions influence perceptual processing and awareness mediated by posterior sensory and association cortices (Vuilleumier & Driver, 2007). More specifically, there is evidence that the prefrontal cortex receives highly processed sensory information and in turn exerts feedback control on posterior association cortices, in order to further modulate representations of affectively relevant information (Miskovic & Schmidt, 2010; Rudrauf et al., 2008). Recent research demonstrated that this modulatory process, indicated by state dependent changes in EEG coherence between prefrontal and temporoparietal regions during the exposure to social-emotional information, relates to the strength or looseness of control over incoming information and, thus, to the impact of the perceptual input on the observer (Papousek et al., 2013, in preparation; Reiser et al., 2012).
Evidence for some temporal stability of prefrontal-posterior coherence changes during the exposure to social-emotional stimuli further supports our interpretation of the present findings: More loose prefrontal-posterior coupling during the exposure to other people's positive affect expressions prospectively predicted greater humor susceptibility which was assessed several weeks later (Papousek et al., 2013). In the present study, potential influences of common situation variance on the results were reduced by obtaining the self-ratings of emotion perception in some temporal distance (one week) from the EEG and ECG recordings. The interpretation of weaker prefrontal-posterior coupling being linked to higher susceptibility to social-emotional information is also in line with reduced prefrontal-posterior coherence being correlated to the personality trait of absorption (Reiser et al., 2012). Absorption is characterised by a readiness for experiences of deep involvement and immersion in environments or events. There is also evidence that the functional connectivity between prefrontal and posterior cortices may be modulated depending on the emotion that is perceived, and that the correlates of individual differences in coupling or de-coupling can be emotion specific (Papousek et al., 2013, in preparation; Reiser et al., 2012). Future studies are necessary to examine the specificity of decreased prefrontal-posterior communication for certain emotions, which might distinguish different psychiatric diseases (e.g., depression, schizophrenia, autism).
Similarly to previous studies in which personality traits and behavioural dispositions were more prominently related to EEG coherence changes in the right than in the left hemisphere (Papousek et al., 2013, in preparation; Reiser et al., 2012), dominance of the right over the left hemisphere was observed in the correlation of coherence changes with individual differences in emotion perception. Higher correlations in the right hemisphere may have been expected because there is broad consensus that the right hemisphere plays a key role in emotion processing, particularly as far as emotion perception is concerned, and for negative emotions such as sadness and fear (Adolphs, 2002; Borod et al., 1998; Najt, Bayer, & Hausmann, 2013; Papousek & Schulter, 2006). In line with this, patients with right hemisphere lesions in anterior parietal regions, but not patients with left hemisphere lesions, showed impairments in emotion perception (Adolphs, Damasio, Tranel, & Damasio, 1996; Adolphs, Damasio, Tranel, Cooper, & Damasio, 2000).
The sudden horrifying events occurring in the film scenes, such as a couple in love sitting on a wall being hit by a car out of control, clearly elicited what in the literature has been described a cardiac "defense" response. Three events were suited for extracting a segment of this response (without responses to other events interfering) which illustrates the most important constituents of the cardiac "defense" response. The results showed that higher scores on perception of other persons' emotions were related to more pronounced cardiac responses (initial acceleration, deceleration, and subsequent acceleration) to the observation of horrifying events occurring to people in the film. Individual differences in emotion perception were not correlated with overall heart rate increases during the entire film or heart rate during a film segment with neutral / mildly positive content. Accordingly, the enhanced heart rate of participants higher on emotion perception shortly before the marked heart rate deceleration was also part of the transient "defense" response. The idea that the cardiac "defense" response reflects heightened external attention and sensory intake (Bradley, 2009; Lang et al., 1997) fits the concept of emotion perception referring to the degree to which one perceives social-emotional information. However, although the proposition of enhanced attention and perceptual processing has been corroborated by empirical evidence, there is no agreement on which component of the defense response is most associated with them (Keil et al., 2010; Vila et al., 2007).
It may also have been expected that distress or the emotional impact of the whole film would be higher in individuals scoring higher on emotion perception. This was not shown with average heart rate increases over the entire 5-min film period. Several possible explanations may account for the failure of the overall heart rate measure to show relationships with individual differences in the perception of other persons' emotions. The film also contained short segments with neutral or mildly positive content (such as a child playing in the garden or a couple in love exchanging endearments), which may have prevented a strong effect on overall heart rate. Moreover, although stressful films and heightened emotional arousal are typically associated with increased heart rates (Fernandez et al., 2012; Weidmann et al., 2009), the marked terrifying events embedded in the film with the associated heart rate decelerations may have in part abolished the increases. Taken together, the present study adds to the evidence that a more fine-grained analysis of transient changes of heart rate can supply information important for interpreting effects that are not available from coarse average values (Lackner et al., 2013). Yet, the finding may also suggest that the investigated perceptual and cognitive processes are more important in explaining individual differences in social-emotional perception than how emotionally affected an individual feels. The failure of the subjectively rated impact of the film to show a relationship to emotion perception may be due to the general weakness of subjective ratings in this context, which are indicative of automatic response tendencies to a lesser degree than physiological measures, because they are influenced by cognitive evaluation (Baumgartner et al., 2006). The retrospective assessment in some temporal distance from the film may have introduced additional error variance.
It is important to note that all effects of self-rated perception of other persons' emotions were independent from individual differences in other interpersonal emotional behaviour (regulation of other persons' emotions) as well as from intrapersonal emotional behaviour (perception and regulation of one's own emotions). Therefore, the effects of the participants' perception of other persons' emotions were specific, that is, they were not, for instance, due to participants' generally more self-confident ratings of their social-emotional behaviour or to their emotion-related competence in general.
Finally, the findings are in line with the conceptualisation of the applied self-report scale, which refers to the individual's susceptibility to other persons' emotions that is based on more automatic, perceptual, and emotional processes rather than effortful cognitive processes (Papousek et al., 2008, 2011; Papousek, Schulter, et al., in press). However, with respect to further integration of the present findings into other research, it should be kept in mind that higher emotion perception according to this concept does not necessarily imply better emotion recognition performance, that is, that other persons' emotions are more correctly identified. Correlations of the interpersonal scales applied in the present study with emotion recognition performance in a widely used paper-and-pencil test were low (Freudenthaler & Neubauer, 2005; Papousek, Schulter, et al., in press). However, the low correlations may also be due to the nature of the performance test which is based on consensus scoring and has been heavily criticised for methodological reasons (Brody, 2004; Freudenthaler & Papousek, in press; Matthews, Roberts, & Zeidner, 2004; Vernon, Villani, Schermer, & Petrides, 2008; Zeidner & Olnick-Shemesh, 2010). Correlations between the self-report social-emotional behaviour scales and emotion recognition performance on a more reliable behavioural performance test have not been examined to date.
In conclusion, the present study showed coherent and well interpretable neurophysiologic and cardiac correlates of self-rated social-emotional perception, which refers to the propensity of perceiving the emotional states of other persons in everday life through observable information such as facial expression and other emotional cues. Higher scores on self-rated emotion perception in everyday life were associated with more pronounced cardiac responses to the observation of horrifying events occuring to people in the film as well as with greater decreases of prefrontal-temporoparietal coupling during viewing the film. While the former finding relates to heightened external attention to and sensory intake of emotionally relevant information, the latter finding relates to loosening of prefrontal modulatory control over incoming social-emotional information. Both processes fit the concept of emotion perception as assessed in the present study, referring to the degree to which one perceives social-emotional information. The fact that these processes are separable supports the notion that, based on established theory and evidence from empirical studies in other fields, neuroscientific research combining different neurophysiological methods may broaden our understanding of the neurobiological basis of social-emotional concepts. As a future prospect, the observed mechanisms may help to explain the high prevalence of emotion perception difficulties and associated social disability in clinical conditions such as schizophrenia, autism, and depression (Harms et al., 2010; Kohler et al., 2010, 2011; Weiss et al., 2006).
Notes
1Additional data were obtained for purposes related to other, non-overlapping research questions. These include genetic data and EEG asymmetries, reported for a larger sample (first experimental session only) in Papousek, Reiser, et al. (in press).
2Analyses in other frequency bands did not reach the significance level.
5. REFERENCES
Addington, J., & Addington, D. (1998). Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophrenia Research, 32, 171-181. doi: 10.1016/S0920-9964(98)00042-5. [ Links ]
Adolphs, R., (2002). Recognizing emotion from facial expressions: psychological and neurological mechanisms. Behavioral and Cognitive Neuroscience Reviews, 1, 21-61. doi: 10.1177/1534582302001001003. [ Links ]
Adolphs, R., Damasio, H., Tranel, D., & Damasio, A. R. (1996). Cortical systems for the recognition of emotion in facial expressions. Journal of Neuroscience, 16, 7678-7687. [ Links ]
Adolphs, R., Damasio, H., Tranel, D., Cooper, G., & Damasio, A. R. (2000). A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. Journal of Neuroscience, 20, 2683-2690. [ Links ]
Aftanas, L. I., Lotova, N. V., Koshkarov, V. I., & Popov, S. A. (1998). Non-linear dynamic coupling between different brain areas during evoked emotions: An EEG investigation. Biological Psychology, 48, 121-138. doi: 10.1016/S0301-0511(98)00015-5. [ Links ]
Bandura, A. (1986). Social foundations of thought and action. Englewood Cliffs, NJ: Prentice Hall. [ Links ]
Borod, J. C., Obler, L. K., Erhan, H. M., Grunwald, I. S., Cicero, B. A., Welkowitz, J., ... Whalen, J. R. (1998). Right hemisphere emotional perception: evidence across multiple channels. Neuropsychology, 12, 446-458. doi: 10.1037/0894-4105.12.3.446. [ Links ]
Baumgartner, T., Esslen, M., & Jäncke, L. (2006). From emotion perception to emotion experience: Emotions evoked by pictures and classical music. International Journal of Psychophysiology, 60, 34-43. doi: 10.1016/j.ijpsycho.2005.04.007. [ Links ]
Bradley, M. M. (2009). Natural selective attention: orienting and emotion. Psychophysiology, 46, 1-11. doi: 10.1111/j.1469-8986.2008.00702.x. [ Links ]
Breslau, N., Bohnert, K. M., & Koenen, K. C. (2010). The 9/11 terrorist attack and posttraumatic stress disorder revisited. Journal of Nervous and Mental Disease, 198, 539-543. doi: 10.1097/NMD.0b013e3181ea1e2f. [ Links ]
Brody, N. (2004). What cognitive intelligence is and what emotional intelligence is not. Psychological Inquiry, 15, 234-238. [ Links ]
Combs, D. R., & Gouvier, W. D. (2004). The role of attention in affect perception: an examination of Mirsky's four factor model of attention in chronic schizophrenia. Schizophrenia Bulletin, 30, 727-738. doi: 10.1093/oxfordjournals.schbul.a007126. [ Links ]
Couture, S. M., Penn, D. L., & Roberts, D. L. (2006). The functional significance of social cognition in schizophrenia: a review. Schizophrenia Bulletin, 32, S44-S63. doi: 10.1093/schbul/sb1029. [ Links ]
Davidson, R. J. (2002). Anxiety and affective style: Role of prefrontal cortex and amygdala. Biological Psychiatry, 51, 68-80. doi: 10.1016/S0006-3223(01)01328-2. [ Links ]
Decety, J., & Moriguchi, Y. (2007). The empathic brain and its dysfunction in psychiatric populations: Implications for intervention across different clinical conditions. BioPsychoSocial Medicine, 1(22), doi:10.1186/1751-0759-1-22. [ Links ]
Decety, J., & Sommerville, J. A. (2003). Shared representations between self and other: A social cognitive neuroscience view. Trends in Cognitive Sciences, 7, 527-533. doi: 10.1016/j.tics.2003.10.004. [ Links ]
Durham, T. W., McCammon, S. L., & Alison, E. J., Jr. (1985). The psychological impact of disaster on rescue personnel. Annals of Emergency Medicine, 14, 664-668. doi: 10.1016/S0196-0644(85)80884-2. [ Links ]
Essl, M., & Rappelsberger, P. (1998). EEG coherence and reference signals: Experimental results and mathematical explanations. Medical and Biological Engineering and Computing, 36, 399-406. doi: 10.1007/BF02523206. [ Links ]
Fernandez, C., Pascual, J. C., Soler, J., Elices, M., Portella, M. J., & Fernandez-Abascal, E. (2012). Physiological responses induced by emotion-eliciting films. Applied Psychophysiology and Biofeedback, 37, 73-79. doi: 10.1007/s10484-012-9180-7. [ Links ]
Freudenthaler, H. H., & Neubauer, A. C. (2005). Emotional Intelligence: The convergent and discriminant validities of intra- and interpersonal emotional abilities. Personality and Individual Differences, 39, 569-579. doi: 10.1016/j.paid.2005.02.004. [ Links ]
Freudenthaler, H. H., & Papousek, I. (in press). The typical and maximum performance of intra- and interpersonal emotion management. In C. Mohiyeddini (Ed.), Emotional relationships: Types, challenges, and physical/mental health impacts. Hauppauge, NY: Nova Science Publishers. [ Links ]
Fries, P. (2005). A mechanism for cognitive dynamics: Neuronal communiction through neuronal coherence. Trends in Cognitive Science, 9, 474-480. doi: 10.1016/j.tics.2005.08.011. [ Links ]
Graham, F. K., & Clifton, R. K. (1966). Heart-rate change as a component of the orienting response. Psychological Bulletin, 65, 305-320. doi: 10.1037/h0023258. [ Links ]
Gross, J., Schmitz, F., Schnitzler, I., Kessler, K., Shapiro, K., Hommel, B., & Schnitzler, A. (2004). Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proceedings of the National Academy of Sciences USA, 101, 13050-13055. doi: 10.1073/pnas.0404944101. [ Links ]
Hagemann, D. (2004). Individual differences in anterior EEG asymmetry: Methodological problems and solutions. Biological Psychology, 67, 157-182. doi: 10.1016/j.biopsycho.2004.03.006. [ Links ]
Harke, K. C., Schlögl, A., Anderer, P., & Pfurtscheller, G. (1999) Cardiac field artifact in sleep EEG. Proceedings of EMBEC '99, 482-483. [ Links ]
Harms, M., Martin, A., & Wallace, G. (2010). Facial emotion recognition in autism spectrum disorders: A review of behavioral and neuroimaging studies. Neuropsychology Review, 20, 290-322. doi: 10.1007/s11065-010-9138-6. [ Links ]
Hautzinger, M., Bailer, M., Worall, H., & Keller, F. (1991). BDI: Das Beck-Depressions-Inventar [Beck Depression Inventory]. Göttingen: Hogrefe. [ Links ]
Haxby, J. V., Hoffman, E. A., & Gobbini, M. I. (2000). The distributed human neural system for face perception. Trends in Cognitive Science, 4, 223-233. doi: 10.1016/S1364-6613(00)01482-0. [ Links ]
Hobson, P. (1993). The emotional origins of social understanding. Philosophical Psychology, 6, 227-249. doi: 10.1080/09515089308573090. [ Links ]
Holmes, E. A., & Bourne, C. (2008). Inducing and modulating intrusive emotional memories: A review of the trauma film paradigm. Acta Psychologica, 127(3), 553-566. doi: 10.1016/j.actpsy.2007.11.002. [ Links ]
Holmes, E. A., Brewin, C. R., & Hennessy, R. G. (2004). Trauma films, information processing, and intrusive memory development. Journal of Experimental Psychology: General, 133, 3-22. doi: 10.1037/0096-3445.133.1.3. [ Links ]
Holmes, E. A., James, E. L., Coode-Bate, T., & Deeprose, C. (2009). Can playing the computer game 'tetris' reduce the build-up of flashbacks for trauma? A proposal from cognitive science. PLoS ONE, 4, e4153. doi:4110.1371/journal.pone.0004153. [ Links ]
Holmes, E. A., James, E. L., Kilford, E. J., & Deeprose, C. (2010). Key steps in developing a cognitive vaccine against traumatic flashbacks: visuospatial Tetris versus verbal Pub Quiz. PLoS One, 5, e13706. doi: 10.1371/journal.pone.0013706. [ Links ]
Izard, C. E. (2001). Emotional intelligence or adaptive emotions. Emotion, 1, 249-257. doi: 10.1037/1528-3542.1.3.249. [ Links ]
Johnstone, T., van Reekum, C. M., Urry, H. L., Kalin, N. H., & Davidson, R. J. (2007). Failure to regulate: Counterproductive recruitement of tow-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience, 27, 8877-8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [ Links ]
Kajantie, E., & Phillips, D. I. (2006). The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology, 31, 151-178. doi: 10.1016/j.psyneuen.2005.07.002. [ Links ]
Keil, A., Bradley, M. M., Ihssen, N., Heim, S., Vila, J., Guerra, P., & Lang, P. J. (2010). Defensive engagement and perceptual enhancement. Neuropsychologia, 48, 3580-3584. doi: 10.1016/j.neuropsychologia.2010.08.007. [ Links ]
Kessels, R. P., Montagne, B., Hendriks, A. W., Perrett, D. I., & de Haan, E. H. (in press). Assessment of perception of morphed facial expressions using the Emotion Recognition Task: Normative data from healthy participants aged 8-75. Journal of Neuropsychology. doi: 10.1111/jnp.12009. [ Links ]
Kohler, C. G., Hoffman, L. J., Eastman, L. B., Healey, K., & Moberg, P. J. (2011). Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Research, 188, 303-309. doi: 10.1016/j.psychres.2011.04.019. [ Links ]
Kohler, C. G., Walker, J. B., Martin, E. A., Healey, K., M., & Moberg, P. J. (2010). Facial emotion perception in schizophrenia: a meta-analytical review. Schizophrenia Bulletin, 36, 1009-1019. doi: 10.1093/schbul/sbn192. [ Links ]
Kopell, N., Ermentrout, G. B., Whittington, M. A., & Traub, R. D. (2000). Gamma rhythms and beta rhythms have different synchronization properties. Proceedings of the National Academy of Sciences USA, 97, 1867-1872. doi: 10.1073/pnas.97.4.1867. [ Links ]
Lackner, H. K., Batzel, J. J., Rössler, A., Hinghofer-Szalkay, H., & Papousek, I. (2013). Multi-time scale perspective in analysing cardiovascular data. Manuscript submitted for publication. [ Links ]
Lang, P. J., Bradley, M. M., & Cuthbert, M. M. (1997). Motivated attention: Affect, activation and action. In P. J. Lang, R. F. Simons, & M. T. Balaban (Eds.), Attention and orienting: sensory and motivational processes (pp. 97-135). Hillsdale, N.J.: Lawrence Erlbaum. [ Links ]
Linden, W., Earle, L., Gerin, W., & Christenfeld, N. (1997). Physiological stress reactivity and recovery: Conceptual siblings separated at birth? Journal of Psychosomatic Research, 42, 117-135. doi: 10.1016/S0022-3999(96)00240-1. [ Links ]
Matthews, G., Roberts, R. D., & Zeidner, M. (2004). Seven myths about emotional intelligence. Psychological Inquiry, 15, 179-196. doi: 10.1207/s15327965pli1503_01. [ Links ]
McPartland, J. C., & Pelphrey, K. A. (2012). The implications of social neuroscience for social disability. Journal of Autism and Developmental Disorders, 42, 1256-1262. doi: 10.1007/s10803-012-1514-z. [ Links ]
Miskovic, V., & Schmidt, L. A. (2010). Cross-regional cortical synchronization during affective image viewing. Brain Research, 1362, 102-111. doi: 10.1016/j.brainres.2010.09.102. [ Links ]
Najt, P., Bayer, U., & Hausmann, M. (2013). Models of hemispheric specialization in facial emotion perception - a reevaluation. Emotion, 13, 159-167. doi: 10.1037/a0029723. [ Links ]
Nygards, M. E. & Sörnmo, L. (1983) Delineation of the QRS complex using the envelope of the ECG. Medical and Biological Engineering and Computing, 21, 538-547. doi: 10.1007/BF02442378. [ Links ]
Papousek, I., & Schulter, G. (1999). Quantitative assessment of five behavioural laterality measures: Distribution of scores and intercorrelations among right-handers. Laterality, 4, 345-362. [ Links ]
Papousek, I., & Schulter, G. (2006). Individual differences in functional asymmetries of the cortical hemispheres. Revival of laterality research in emotion and psychopathology. Cognition, Brain, Behavior, 10, 269-298. [ Links ]
Papousek, I., Freudenthaler, H. H., & Schulter, G. (2008). The interplay of perceiving and regulating emotions in becoming infected with positive and negative moods. Personality and Individual Differences, 45, 463-467. doi:10.1016/j.paid.2008.05.021. [ Links ]
Papousek, I., Freudenthaler, H. H., & Schulter, G. (2011). Typical performance measures of emotion regulation and emotion perception and frontal EEG asymmetry in an emotional contagion paradigm. Personality and Individual Differences, 51, 1018-1022. doi:10.1016/j.paid.2011.08.013. [ Links ]
Papousek, I., Mosbacher, J., Fink, A., Schulter, G., & Weiss, E. M. (in preparation). Loose control of social-emotional information: Affective processing in positive schizotypy. Manuscript in preparation. [ Links ]
Papousek, I., Schulter, G., Lackner, H. K., Samson, A. C., & Freudenthaler, H. H. (in press). Experimentally observed responses to humour are related to individual differences in emotion perception and regulation in everyday life. Humor: International Journal of Humor Research. [ Links ]
Papousek, I., Reiser, E. M., Schulter, G., Fink, A., Holmes, E. A., Niederstätter, H., ... Weiss, E. M. (in press). Serotonin transporter genotype (5-HTTLPR) and electrocortical responses indicating the sensitivity to negative emotional cues. Emotion. doi: 10.1037/a0033997. [ Links ]
Papousek, I., Reiser, E. M., Weiss, E. M., Fink, A., Samson, A. C., Lackner, H. K., & Schulter, G. (2013). State-dependent changes of prefrontal-posterior coupling in the context of affective processing: Susceptibility to humor. Cognitive, Affective, and Behavioral Neuroscience, 13, 252-261. doi: 10.3758/s13415-012-0135-5. [ Links ]
Perez, N., Fernandez, M. C., Vila, J., & Turpin, G. (2000). Attentional and emotional modulation of cardiac defense. Psychophysiology, 37, 275-282. doi: 10.1111/1469-8986.3730275. [ Links ]
Phillips, M. L., Ladouceur, C. D., & Drevets, W. C. (2008). A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13, 833-857. doi: 10.1038/mp.2008.65. [ Links ]
Puce, A., Allison, T., Bentin, S., Gore, J. C., & McCarthy, G. (1998). Temporal cortex activation in humans viewing eye and mouth movements. Journal of Neuroscience, 18, 2188-2199. [ Links ]
Ramirez, I., Sanchez, M. B., Fernandez, M. C., Lipp, O. V., & Vila, J. (2005). Differentiation between protective reflexes: cardiac defense and startle. Psychophysiology, 42, 732-739. doi: 10.1111/j.1469-8986.2005.00362.x. [ Links ]
Reiser, E. M., Schulter, G., Weiss, E. M., Fink, A., Rominger, C., & Papousek, I. (2012). Decrease of prefrontal-posterior EEG coherence: Loose control during social-emotional stimulation. Brain and Cognition, 80, 144-154. doi: 10.1016/j.bandc.2012.06.001. [ Links ]
Rudrauf, D., David, O., Lachaux, J. P., Kovach, C. K., Martinerie, J., Renault, B., & Damasio, A. (2008). Rapid interactions between the ventral visual stream and emotion-related structures rely on a two-pathway architecture. Journal of Neuroscience, 28, 2793-2803. doi: 10.1523/JNEUROSCI.3476-07.2008. [ Links ]
Schellberg, D., Besthorn, C., Klos, T., & Gasser, T. (1990). EEG power and coherence while male adults watch emotional video films. International Journal of Psychophysiology, 9, 279-291. doi: 10.1016/0167-8760(90)90060-Q. [ Links ]
Schnitzler, A., & Gross, J. (2005). Normal and pathological oscillatory communication in the brain. Nature Review Neuroscience, 6, 285-296. doi: 10.1038/nrn1650. [ Links ]
Scholten, M. R., Aleman, A., Montagne, B., & Kahn, R. S. (2005). Schizophrenia and processing of facial emotions: sex matters. Schizophrenia Research, 78, 61-67. doi: 10.1016/j.schres.2005.06.019. [ Links ]
Schuster, M. A., Stein, B. C., Jaycox, L. H., Collins, R., Marshall, G. B., Elliott, M. N., ... Berry, S. H. (2001). A national survey of stress reactions after the September 11, 2001, terrorist attacks. New England Journal of Medicine, 345, 1507-1512. doi: 10.1056/NEJM200111153452024. [ Links ]
Srinivasan, R., Winter, W. R., Ding, J., & Nunez, P. L. (2007). EEG and MEG coherence: Measures of functional connectivity at distinct spatial scales of neocortical dynamics. Journal of Neuroscience Methods, 166, 41-52. doi: 10.1016/j.jneumeth.2007.06.026. [ Links ]
Steingrüber, H., & Lienert, G. (1971). Hand-Dominanz-Test. Göttingen: Hogrefe. [ Links ]
Steketee, G. S., & Chambless, D. L. (1992). Methodological issues in prediction of treatment outcome. Clinical Psychology Review, 12, 387-400. doi: 10.1016/0272-7358(92)90123-P. [ Links ]
Vasey, M. W., & Thayer, J. F. (1987). The continuing problem of false positives in repeated measures ANOVA in psychophysiology: a multivariate solution. Psychophysiology, 24, 479-86. doi: 10.1111/j.1469-8986.1987.tb00324.x. [ Links ]
Vernon, P. A., Villani, V. C., Schermer, J. A., & Petrides, K. V. (2008). Phenotypic and genetic associations between the big five and trait emotional intelligence. Twin Research and Human Genetics, 11, 542-530. doi: 10.1375/twin.11.5.524. [ Links ]
Vila, J., Perez, M. N., Fernandez, M. C., Pegalajar, J., & Sanchez, M. (1997). Attentional modulation of the cardiac defense response in humans. Psychophysiology, 34, 482-487. doi: 10.1111/j.1469-8986.1997.tb02393.x. [ Links ]
Vila, J., Guerra, P., Munoz, A., Vico, C., Viedma del Jesus, M. I., Delgado, L. C., ... Rodriguez, S., (2007). Cardiac defense: from attention to action. International Journal of Psychophysiology, 66, 169-182. doi: 10.1016/j.ijpsycho.2007.07.004. [ Links ]
Vuilleumier, P., & Driver, J. (2007). Modulation of visual processing by attention and emotion: Windows on causal interactions between human brain regions. Philosophical Transactions of the Royal Society B, 362, 873-855. doi: 10.1098/rstb.2007.2092. [ Links ]
Weidmann, A., Conradi, A., Gröger, K., Fehm, L., & Fydrich, T. (2009). Using stressful films to analyze risk factors for PTSD in analogue experimental studies - which film works best. Anxiety, Stress, and Coping, 22, 549-569. doi: 10.1080/10615800802541986. [ Links ]
Weisenbach, S. L., Rapport, L. J., Briceno, E. M., Haase, B. D., Vederman, A. C., Bieliauskas, L. A., ... Langenecker, S. A. (in press). Reduced emotion processing efficiency in healthy males relative to females. Social, Cognitive and Affective Neuroscience. doi: 10.1093/scan/nss137. [ Links ]
Weiss, E. M., Kohler, C. G., Nolan, K. A., Czobor, P., Volavka, J., Platt, M. M., ... Gur, R. C. (2006). The relationship between history of violent and criminal behavior and recognition of facial expression of emotions in men with schizophrenia and schizoaffective disorder. Aggressive Behavior, 32, 187-194. doi: 10.1002/ab.20120. [ Links ]
Whittle, S., Yücel, M., Yap, M. B. H., & Allen, N. B. (2011). Sex differences in the neural correlates of emotion: Evidence from neuroimaging. Biological Psychology, 87, 319-333. doi: 10.1016/j.biopsycho.2011.05.003. [ Links ]
Zeidner, M., & Olnick-Shemesh, D. (2010). Emotional intelligence and subjective well-being revisited. Personality and Individual Differences, 48, 431-435. doi: 10.1016/j.paid.2009.11.011. [ Links ]