Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
International Journal of Psychological Research
Print version ISSN 2011-2084
int.j.psychol.res. vol.9 no.1 Medellín Jan./June 2016
Potential Biomarkers in personality disorders: current state and future research
Potenciales Biomarcadores en Trastornos de la Personalidad: estado actual y trabajo futuro
Manuela Valenciaa and Jorge Mauricio Cuartas Ariasa,b,*
a Investigador asociado, Grupo de Investigación Salud Comportamental y Organizacional, Universidad de San Buenaventura, Medellín, Colombia.
b Facultad de Psicología, Universidad de San Buenaventura, Medellín, Colombia.
* Corresponding author: Jorge Mauricio Cuartas Arias, Facultad de Psicología, Universidad de San Buenaventura, Medellín, Colombia. Email address: mauricio.cuartas@usbmed.edu.co.
Article history: Received: 15-09-2015 Revised: 31-10-2015 Accepted: 01-12-2015
ABSTRACT
This review article presents the most appropriate strategies for examining the phenotype for personality disorders. At present there are many neurobiological and molecular studies that suggest a genetic predisposition to different traits representative of expressed personality disorders. Nonetheless, it has not been possible to accurately and successfully replicate such results due to some difficulties regarding the sensitivity, specificity and validity of the clinical evaluation methods, and the size and type of the chosen population and experimental designs used for research. Unfortunately, diagnoses done in psychiatry and psychology have a classification system based on the prevalence and intensity of symptoms and do not take into account the etiology, neurobiology, epidemiology, genetics, and drug responses. On the other hand, explaining the phenomenology of personality disorderes and how genes work together to express this phenotype implies a revision of the chaos theory, addressing the connection between neurodevelopment, significantly stressful events during early childhood and epigenetic modifications in DNA related to stochastic events which may contribute to the development of normal or abnormal behavior.
Key words: personality disorders, ERPs, biomarkers, candidate genes.
RESUMEN
Este artículo de revisión presenta las estrategias más apropiadas para el estudio de endofenotipos en los trastornos de personalidad. Actualmente existen muchos estudios neurobiológicos y moleculares que sugieren una predisposición genética a diferentes rasgos representativos expresados en dichos trastornos. No obstante, la replicación exitosa de tales resultados no ha sido posible, debido a dificultades relacionadas a la sensibilidad, especificidad y validación de los métodos implementados en las evaluaciones clínicas; el tamaño y tipo de poblaciones elegidas, y los diseños experimentales implementados. Desafortunadamente, los diagnósticos hechos en psiquiatría y psicología cuentan con un sistema de clasificación basado en la prevalencia y la intensidad de los síntomas, sin tener en cuenta la etiología, neurobiología, epidemiología, genética y la respuesta a los medicamentos. Por otra parte, explicar la fenomenología de los trastornos de personalidad y como los genes expresan el fenotipo, implica una revisión de la teoría del caos, direccionándola a la conexión entre en neurodesarrollo, eventos estresantes significativos durante la infancia temprana y modificaciones en el ADN asociados a eventos estocásticos que contribuyen al desarrollo de conductas normales y anormales.
Palabras clave: trastornos de personalidad, ERPs, biomarcadores, genes candidatos.
1. NTRODUCTION
The costs in terms of public health for personality disorders in developing countries have not been established yet. However, we do know that conducts caused by these disorders decreases the quality of life and a significant loss in human capital. Personality disorders are a serious mental health condition responsible for individual history for criminal arrest, interpersonal violence and suicidal behaviors. Additionally, personality disorders are associated with medical morbidity and mortality (Samuels, 2011).
2. PREVALENCE OF PERSONALITY DISORDERS
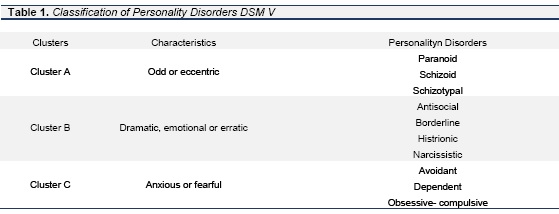
Personality disorders (PD) are described by the the American Psychiatric Association and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) as "an enduring pattern of inner experience and behavior that deviates markedly from the expectations of the individual's culture, is pervasive and inflexible, has an onset in adolescence or early adulthood, is stable over time, and leads to distress or impairment." The DSM-5 identifies ten PD grouped into three clusters based on descriptive similarities. See table 1. Data submitted by The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), estimated that 15% of adult North Americans fulfill the criteria for a personality disorder. Conforming to the DSM-5 the prevalence for disorders in cluster A is 5.7%, cluster B, 1.5% for cluster C, 6.0% and 9.1% for any personality disorder. (DSM-5, 2013). See table 1.
The occurrence of antisocial personality disorder (ASPD) worldwide fluctuates between 1 and 3% (Blanco et al., 2010; Goldstein & Grant, 2009) a number similar to the schizophrenia or bipolar affective disorder type. However, the biomedical studies are enormous (Cuartas Arias et al., 2011) for last ones in comparison with ASPD; this means that the negative impact that entails ASPD for individuals and societies has not yet been evaluated enough and this constitutes a harmful phenomenon in the development and collective mental health, as well as its high cultural and social cost. The rates of occurrence vary according to the culture and context in which the patterns of behavior characteristic for PD are established. Therefore, there is no general consensus worldwide. Generally PD has a chronic course, although there is evidence of a decrease in bizarre behaviors after the age of 40, which allows for an important variation in the decrease and severity of the symptoms along with the increase of the age (Boutwell & Beaver, 2008; Huchzermeier et al., 2008).
3. THE CONCEPT OF BIOMARKER
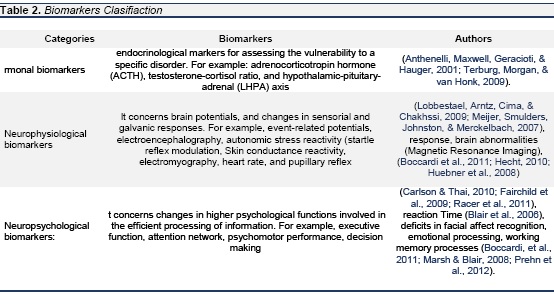
It is important to take into account that a biomarker is just an indicator of biological processes since it does not play a causal role within the course of a disease and, thus, it may be akin to a biological signal which points towards the presence of a specific pathology - for example, melatonin levels as a biomarker for schizophrenia (Carmeli, Knyazeva, Cuenod, & Do, 2012; Morera, Abreu-Gonzalez, Henry, & Garcia-Hernandez, 2009). According to this, it is usual that biomarkers be measurable substances - for instance, macromolecules like proteins - which are used to assess the presence and risk of a particular organic disorder. Hence, a general notion about what constitutes a biomarker is to regard it as an objectively measurable feature which can assess normal and pathological conditions so that it is used as an indicator of the presence of and degree at which a biological process is directly linked to clinical products and symptoms of a particular disease (Courtet, Gottesman, Jollant, & Gould, 2011; DiLalla & Gottesman, 1991). It is remarkable to highlight three points that Ritsner and Gottesman (2009) expose in order to determine a biomarker's efficacy, namely, 1) a biomarker must depict a basic physiopathological process and detec a fundamental characteristic of the disorder; 2) a biomarker must be specific for a pathology in contrast with others; and 3) a biomarker must not consist in clinic symptomatology (Bertelsen, 2011). In addition to the general definition that has been given of a biomarker, it should be noted that depending on the field of application there may be several sorts of biomarkers (Ritsner & Gottesman, 2009, pág. 6). Thereby, in accordance with the purpose of this article we classify biomarkers as depicted in table 2.
3.1 Biomarkers and Endophenotypes
It should be remarked the difference which exist between biomarkers, and another concept whose function is also, partly, to give an account of biological processes: the endophenotype. This term, also known as an intermediate phenotype, was originally treated as an internal phenotype that could be used as a clinic variable far more rigorous in the studied phenotype, thus, being more reliable to genetic searches. Some of the most outstanding characteristics belonging to endophenotypes are depicted in table 3. However, the most important component of an endophenotype which distinguishes it from a biomarker is that it is intended to be an intermediary variable in the causal course of pathologies (Benjamin, Ebstein, & Belmaker, 2001, pág. 54) through the identification of genetic polymorphisms which are associated with particular effects in neuronal function, physiology and neurocognition (Glenn, 2010). Hence, it is this causal role which differentiates endopehnotypes from biomarkers - the latter being only risk indicators of biological processes, but not playing any causal role (Ritsner & Gottesman, 2009, pág. 7)Having carried out this distinction between endophenotypes and biomarkers it is possible to conclude that a biomarker merely points to the physiological expression of particular morphological, biochemical or molecular conditions which are closely related with the state of a disease or syndrome. Therefore, a candidate biomarker must be sensible, specific, reversible, and replicable in pharmacodynamics. Nevertheless, although a biomarker's expression may vary in short time intervals (from hours to days), it is plain over time.
3.1.1 Endophenotypes in PD
The study of endophenotypes provides evidence to identify the basis genotypes, which are strongly correlated with the traits of the PD. Moreover, these basis can provide information about the susceptibility to those disorders. As previously mentioned, studies designed with twins, families and adoptive families as well, suggests that a genetic component is involved not only for personality but also for PD (Siever, 2005). Due to the stability of the traits in PD, the studies based on endophenotypes are facilitated. These traits and interactive dimensions can be formulated into the laboratory for behavioural and neurobiological endophenotypes, which provide close understanding of underlying genotypes (Siever, 2005). Some of the clinical dimensions studied under the paradigm of endophenotypes are impulsivity, aggression, emotional information processing, attention, working memory, among others. Adittionally, the electrophysiological measures can be applied to diagnosis, prevention and treatment of the PD or various clinical conditions (Kamarajan & Porjesz, 2015).
3.2 Biomarkers and Development in PD
Obstetric complications can become markers of vulnerability in the expression of psychopathic features; this condition could originate neurological damages and susceptibility to the development of PD. On the subject, Allen and colleagues evaluated in 1998 some such factors associated with pregnancy and birth labor as family relations, prematurity and the consumption of tobacco and observed that obstetric complications were associated with the occurrence of an agitated behavior, impulsivity and antisocial acts. Some obstetric complications were related to the mother's emotional disorders during pregnancy; additionally, neonatal anoxia is a significant factor of risk of ASPD disorder (Allen, Lewinsohn, & Seeley, 1998; Wermter et al., 2010). Raine and his group carried out a longitudinal research evaluating the relations between obstetric complications and the subsequent occurrence of psychopathology in a sample of 4269 newly born individuals.
Some of the variables such as socioeconomic situation, mental characteristics of the mother, as well as rejection from the mother (attempt to abort, the abandonment of the child) were also analyzed, showing that the rejection from the mother, summed up to some obstetric complications were associated significantly to violent acts in the future (Raine, Brennan, & Mednick, 1997). In other studies prenatal problems such as preeclampsia, umbilical cord prolapse and prolonged or induced birth labor, have been associated with the occurrence of violent behavior between the age of 6 and 17 years (Allen, et al., 1998; Bahmanyar, Montgomery, Weiss, & Ekbom, 2008). On the other hand, consumption and exposure to toxic substances could be considered as a potential endophenotype for the development of aggressive and impulsive forms of behavior, due to the fact that the fetus is affected in its normal development if psychoactive substances are consumed during pregnancy. The implications of tobacco consumption during pregnancy were analyzed by Fergunsson and collaborators in their prospective research during 18 years with 1265 children, in which they found that the excessive consumption of tobacco is associated with the occurrence of a higher number of paidopsychiatric disorders, such as dissocial disorder and alcohol consumption (Fergusson, Woodward, & Horwood, 1998).
These findings were supported by those of Brennan who found in a retrospective study of a historical cohort of 4169 individuals which ones had committed antisocial acts, a positive relation between the quantity of consumed tobacco and violent acts during adolescence and adult age (Brennan PA, 1999). The consumption of tobacco and maternal use of alcohol during pregnancy could have implications on the noradrenergic, serotonergic and dopaminergic circuits, which have a negative influence on brain and development (Brookes et al., 2006; Wermter, et al., 2010).
3.3 Biomarkers in receptors and neurotransmitters for PD
The Biogenetic Amines have been a research focus in view of their involvement in the presence of violent behavior. In general terms, serotonin (5-HT) has an inhibitive action in the brain (Daw, Kakade, & Dayan, 2002); the serotonergic dysfunction has been frequently associated with aggressive behavior in animals and human beings.
In primates, low concentrations of 5-HIAA have been related to recidivist behavior of aggression and impulsivity (Dongju Seo, 2008; Fairbanks, Melega, Jorgensen, Kaplan, & McGuire, 2001; Mehlman et al., 1994), and to poor impulse control (Mehlman, et al., 1994). In contrast, the low concentrations of 5-HIAA have repeatedly been associated with aggression through lifetime in individuals with some kind of mental disorder, either violent suicide, impulsivity or recidivist homicidal acts (Dongju Seo, 2008; Moore, 2002). In accordance with these findings, Moore and collaborators carried out a meta-analysis including 20 different studies in which it is evident that low levels of serotonin contributed to impulsive behavior, which was even correlated to some kind of crime. Different studies have demonstrated the association of PD with a decrease of the function of the serotonergic system. 5-HT is perhaps one of the neurotransmitters that has been most studied since it modulates a series of biological and psychological functions such as the endocrine regulation, the state of mind, the libido, the regulation of aggression, and so on. Moreover, the 5-HT receptors have repeatedly been associated with impulsivity and aggressive behavior in different types of animal models, in which researchers have tried to recognize the type of aggression it expresses Although the relations between the hypofunction of serotonin and the impulsive behavior have been consistent with the contributions of clinical neurosciences, there are still few studies that detail this relation. It has been suggested that the serotonergic hypofunction and its strong association with impulsive behavior could have a genetic base such that the deficiency in the serotonin function could constitute a neurochemical biomarker of impulsive behavior (Dongju Seo, 2008). Another molecule that has been interesting and that could end up as a biomarker in PD refers to free l-tryptophan plasmatic levels of tryptophan, amino acid, precursor of 5-HT, which is co-related with high rates on the scales of aggression (Moller et al., 1996). So far, free l-tryptophan and competing amino acids have could be useful in early indicators for the development of impulsive behavior (Virkkunen et al., 2003).
Likewise, the variation in MAO-A (monoamine oxidase A), has been co-related with the reduction of aggression in mice, probably as a result of the increase in the levels of 5-HT (Nelson & Chiavegatto, 2001). It is known that a reduction of the activity of the platelet MAO-A is related with impulsivity and PD and that it is also associated with 5-HAA in LCR, thus presenting an inverse correlation with rates at scales that search for sensations and impulsivity (Schalling, Asberg, Edman, & Oreland, 1987). In addition, recent studies have replicated of gene x environment (G x E) interactions involving the MAO-A, indicating that activity MAO-A genetic variant who were more associated to PD traits like as hostility and impulsivity (Fergusson, Boden, Horwood, Miller, & Kennedy, 2011; Philibert et al., 2011; Reti et al., 2011)
3.4 Biomarkers of Emotional Expression
The implications of facial expression were initially formulated by Charles Darwin in 1872; later, a lot of researches have suggested that the basic emotions in human beings can be identified through facial expressions and brain activation (Panksepp, 1992; Ortony Turner &, 1990). Regarding individuals with PD, studies with PET have shown that a low level of activation in the ventromedial prefrontal area could influence a brain circuit which depicts a biomarker in which there are impairments in the monitoring of feelings and emotions of oneself and others. Supporting this, it is suggested that in these individuals the contra-lateral region of the right hemisphere is related to their failure to process emotional expressivity which explains why their faces seem not to be altered when emotional asymmetric differences occur. Facial asymmetries are produced by several anatomical, physiological, neurological, psychological, pathological and socio-cultural factors. From neuropsychology it has been pointed that facial expressions correspond to asymmetries in brain functions (Borod, Haywood, & Koff, 1997). Thereby, the fact that from psychosocial vulnerability factors several biomarkers related to neuronal circuits (involved in facial asymmetries) may be derived is remarkable (Borod, et al., 1997). Particularly, the emotional deprivation that implicates a limit to the possibilities of establishing relations between affection and empathy -a condition that is co-related with the presence of psychopathic features - could lead to an emotional malfunction that is expressed by facial configurations that are controlled by both brain hemispheres (Birbaumer et al., 2005; Caspi et al., 2002; Dolan & Fullam, 2006; Kosson, Kosson, Lorenz, & Newman, 2006; Marsh & Blair, 2008). Corroborating this, Facial analyses show a reduction in the activation of the right brain hemisphere and the prefrontal ventromedial cortex, and a deficit in the function of the amygdala (which is associated with recognition and perception of expression of fear and sadness).
4. NEUROPHYSIOLOGICAL BIOMARKERS FOR PD
Psychophysiology (electroencephalogram and event-related potentials), positron emission tomography (PET), single photon emission computed tomography (SPECT) and magnetic resonance imaging (MRI), all of them have allowed to decipher the brain areas implicated in PD. These methods help detect the activation of structures, in response to physiological or cognitive tasks and a deficit in the emotional reactivity has been reported. Several studies have reported reduced P3 amplitude in bipolar disorder and ASPD, also, this is a multi-determined component indexing attention and working memory processes related to executive function. These data support the deficient startle potentiation and suggesting that ASPD traits are associated with distinctive information-processing characteristics as indexed by P3 amplitude. (Anderson, Stanford, Wan, & Young, 2011; Carlson & Thai, 2010). In a recent study Hall, M. et al. (2015), conducted a genomewide association analysis of electrophysiological endophenotypes study for some PD, schizophrenia and psychotic bipolar disorder. They identified a region on chromosome 14 that was significantly associated with sensory gating. Peak of the Single nucleotide polymorphism (SNP) (rs10132223, P= 1.27* [10]ˆ (-9)). In the polygenic risk scores between genetic components of event - related potentials (ERP) and mood and psychotic disorders, they found that patients with higher load of esquizofrenia risk alleles had reduced gamma responses while patients with higher load of bipolar disorder risk alleles had smaller P3 amplitude. To research efficiently into a biomarker (related with the emotional imbalance in personality disorder) which indicates the presence of emotional stable or instable devices to face situational stress, some other physiological measurement must be used, namely, such an interesting variable as the electromyography evaluation (Herpertz et al., 2001; Lang, Greenwald, Bradley, & Hamm, 1993). The electrodermal response is an indicator of emotional arousal and of the spontaneous responses of alert present in the processing of emotional stimuli. The evaluation of this endophenotype has evidenced that it is difficult to respond adequately to aversive stimuli and balance between a stimulus' positive and negative features in PD.
Undoubtedly, one of the areas of the brain cortex most implicated in the irregular expression of PD is the orbitofrontal cortex; damages in this area implicate behavior changes and neurocognitive malfunction (Stevens, Kaplan, & Hesselbrock, 2003). With the use of evoked potentials, a decrease of the amplitude of the P300 wave has been observed in the frontal electrodes of individuals with high rates of aggressiveness (Bauer & Hesselbrock, 2001 ; Benning, Patrick, & lacono, 2005; Kostandov, Tal'tse, Zakharova, & Vazhnova, 1994; Patrick et al., 2006). One of the most consistent approximations for the studies of endophenotypes related with PD has been the use of neuroimage tools, which have associated impulsivity with a reduction of the volume of the prefrontal grey substance and with a decrease in the perfusion of the temporal cortex and in the ventrolateral area at the right of the prefrontal cortex.
A neural dysfunction and white matter microstructural abnormality in the inferior frontal regions has been associated with PD (Hoptman, 2003; Narayan et al., 2007; Sundram et al., 2012). Through the use of the PET it has been possible to associate a decrease in brain fluid on the left side of the orbitofrontal cortex, the right side of the ventral cingulated area and the bilateral temporal poles, with violent behavior (Dougherty et al., 1999; Hoptman, 2003). With the use of the SPECT, patterns of behavior have been found characteristic of some PD, e.g; ASPD which is associated with a prefrontal hypoperfusion and an increase in blood fluid in the anteromedial frontal region and the left zone of the basal gland in the lymphatic system (Amen, Stubblefield, Carmicheal, & Thisted, 1996). Likewise, some studies reported a significant association with a decrease in gray matter temporal brain regions, in particular in the right superior temporal gyrus, this findings supporting the hypothesis that a disturbed frontotemporal network is critically involved in the pathogenesis of general PD (Muller et al., 2008; Soderstrom et al., 2002). As a result of these multiple findings a hypothesis has been suggested to explain the low functioning of some brain systems in psychopathology: it seems that these individuals, in order to compensate for deficits in the prefrontal lymphatic circuit, use the dorsolateral prefrontal cortex, which explains the deficits in the emotional processing and the execution of basic cognitive tasks. Up to now, one of the central problems of neuroimaging studies is co-morbidity and therefore the differential identification of affected brain regions. Specifically for PD, associated with alcoholism, drug addiction, PD in general could show significant correlations in the same regions of interest. At present, neuroimaging studies for quantitative features that include clinical dimensions and diagnostic evolution have not had conclusive results for the etiopathology of these disorders. Nevertheless, the use of neuroimage and psychophysiological tools help configurate diagnostic subtypes that best decipher the etiology of the syndrome.
5. BEHAVIORAL BIOMARKERS RELATED TO NEUROCHEMICAL AND HORMONAL VARIABLES IN PD
Initially, impulsive behavior has been associated with endocrine pathologies such as Cushing's syndrome, hyperandrogenism, hyperthyroidism, hypoglycemia, and premenstrual syndrome, but these findings are still controversial. In this regard, it has been suggested that gender's physiological variations could be the outcome of a prenatal differentiation in the preoptic area of the hypothalamus due to an influx of androgens. Thus, several molecular circuits has been reported in which gonadal hormones are responsible for gender differences, there being biomarkers such as sexuality, social dominance, disinhibition and aggressiveness which are related to testosterone in animals (Onyekwere & Ramirez, 1994). Indubitably, aggressive behavior involves a complex variety of interactions among several hormonal systems such as the gonadal - with independent activations of androgens and estrogens -, the suprarenal, hypothalamus-hypophyseal and even the Luteinizing-hormone releasing hormone (LHRH) -which stimulates the production of sexual hormones both in men and women (Ramirez & Delius; 1979. Ramirez & Carrer, 1982). Therefore, from investigations based on androgens, specific biomarkers could emerge for hormonal systems, this being quite helpful in order to predict PD such ASPD. For example, a tendency to aggressiveness, visuo-spatial abilities, and exitability are behavioral characteristics in which androgens are generally involved; the opposite occurs with estrogens. The main thesis which supports the assessment of biomarkers associated with testosterone comes from the observation of male animals in the majority of species. Thus, it is affirmed that high levels of testosterone determine the degree of aggression. Additionally, over the pubertal development a hormonal increase is observed basically in in the hypothalamus-gonadal and hypothalamus-suprarenal systems. This condition explains why some PD traits in many adolescents are related to a decrease of the gonadal steroids and an increase of estrogens. Hence, it is possible to suggest that androgens are involved with biomarkers related to aggressive. (Halpern, Udry, Campbell, & Suchindran, 1993;). On the other hand, two mechanisms could explain why testosterone triggers aggressive responses: one is the afferent path for the androgens which implicates a relative sexual dimorphism of the estrogens in regard with sensibility of the tissue to which they are directed. The other one is the afferent path for estrogens in which an absolute sexual dimorphism is associated with the response ability of estrogens to generate aggression. Consequently, due to testosterone's triggering effect in aggression and impulsivity, several biomarkers can be suggested which are related to PD, although, up to now, studies regarding variations in testosterone as an activating factor in aggressive or impulsive behavior are polemic, and there exists a tendency to rule out that aggression and impulsivity levels are modulated specifically by this hormone.
Moreover, the 5-HydroxyindoIeacetic Acid ( ), which is a metabolized product of the degradation of the 5-HT, has been found in minor concentrations in the cerebrospinal fluid (LCR) of patients with PD (Constantino, Morris, & Murphy, 1997). The low levels of 5-HIAA in LCR (especially in the cortex and the raphe nucleus) seem to constitute a molecular signal linked to aggression, irritability, hostility and criminal activity which are not influenced by the ingestion of either medical drugs or psychotropic drugs (Soderstrom, Blennow, Manhem, & Forsman, 2001). Thus suggests that irritability, hostility and impulsivity activity could stand for biomarkers in PD so long as they are related to Low levels low levels of 5-HIAA in LCR. Also, biomarkers derived from suprarenal hormone's expression could be considered as indicators of personality disorder. In this regard, it has been proposed that hormones of the hypophysic-suprarenal axis - which involve the suprarenal cortex - through corticosterone (which stimulates the secretion of cortisol) and through the catecholamines adrenaline, noradrenaline, are associated with aggression and impulsivity so that these traits might be emergent biomarkers regarding those circuits. On the other hand, the glucocorticoids can inhibit the thyrotropin-releasing hormone (TRH) and the stimulating hormone of the thyroid (TSH), according to which individuals with higher levels of TRH tend to show characteristic markers of PD and conditions such as suicidal attempts and violent behaviors (Sher et al., 2005). In general, cortisol's main function is to regulate biological stress. Specifically, individuals who present low levels of cortisol show chronic violence, which is closely related to PD (Pajer, Gardner, Rubin, Perel, & Neal, 2001). Additionally, it has been registered that there exists a positive correlation between the excretion of adrenaline and some PD such ASPD. These several biomarkers could derive from here. Furthermore, it has been shown that there is a close correlation between high levels of noradrenaline in predatory mammals and their aggressive and impulsive behaviors (Ramirez, & Andreu, 2006). In contrast, Prolactin has evidenced a minor response to the agonists of serotonin in individuals with PD (Dolan, Deakin, Roberts, & Anderson, 2002), which constitutes potential biomarkers in psychopathology (Dolan, Deakin, Roberts, & Anderson, 2002).
6. NEUROPSYCHOLOGICAL BIOMARKERS AND ASSESSMENT TOOLS
In PD there are neuropsychological evidences which suggest biomarkers related to an increase in cortical arousal which hinders certain psychological abilities regarding thinking skills, in particular the interpretation of external events, in accordance with a deficit of basic mental processes, such as attention, concentration and memory. Up to now, the increasing number of researches has suggested that deficits in emotional processes, patent in PD, could be a biomarker related to attention disorders which have a neuropsychological origin (Caspi, et al., 2008; Finger et al., 2008; Herpertz et al., 2008; Malterer, Glass, & Newman, 2008). Furthermore, attention disorders linked to the processing of peripheral information and to impairments in executive function associated with work memory may be putative biomarkers with a close relation to the prefrontal cortex (Berman & Coccaro, 1998; Coccaro, Kavoussi, Berman, & Lish, 1998; Sadeh & Verona, 2008).
The assessment of biomarkers related to executive function regards the analysis of complex cognitive components involved in the individual's information processing. Some components may include abstract behavior, ethics, planning, self-regulation, ability to start, continue and stop planning complex sequences of behavior and the regulation of impulses. Some of the tools that offer a good sensitivity and allow the execution of neutral co-relations, adjusted to psychopathy, are the NEUROPSI test (Ostrosky-Solis et al., 2007) which evaluates in detail the processes of attention and memory (with 27 sub-tests) and allows to obtain independent and global rates of both functions. Also, to measure the frontal and executive functions, Flores and Ostrosky-Solien developed in 2008 a diagnostic set of instruments that includes 15 sub-tests for monitoring the performance of tasks related with orbital, dorsolateral and prefrontal integrity of both brain hemispheres.
All this shows that the assessment of executive functions is a fundamental tool in order to derive biomarkers related to cognitive and neurological variables for PD. Thus, several researchers have used alternative tests that help considerably to explain the neuronal correlates which are involved in this disorder. Some of those test are the Wisconsin Card Sorting Test (WCST) (Easton, Sacco, Neavins, Wupperman, & George, 2008; Eling, Derckx, & Maes, 2008) which evaluates the capacity of abstraction and cognitive flexibility (associated with a dorsolateral substrate) such that it explains apathy, irritability, and mainly the deficits in task-planning. The deficits in planning have been a controversial biomarker since some studies involve it in PD. The go-no-go test allows discriminating visual-spatial skills, associated with orbifrontal deficits which have been observed in subjects with PD and which suggest the inability to inhibit impulsive responses as a possible biomarker. Moreover, the Stroop Test has shown orbifrontal alterations which are related neither with deterioration of the dorsolateral cortex nor with the cingulated cortex, thus conferring a neuropsychological biomarker of brain deterioration in these individuals (Blair et al., 2006; Hiatt, Schmitt, & Newman, 2004).
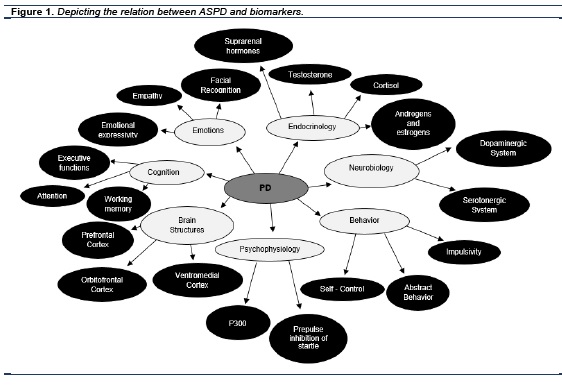
In order to make a synthesis encompassing what has so far been mentioned, the following figure is intended to depict the relation between PD and different types of biomarkers. See figure 1.
7. CONCLUSIONS
PD spectrum incorporates certain traits subtypes such as dichotomic features. Unfortunately, current classifications that categorize PD lack a solid neurobiological correlate to delineate or differentiate the subtypes. The use of biomarkers to delineate personality disroders is fundamental to explain the etiology of these patterns of behavior as well as to identify their subtypes, which could end up having an impact on psychotherapy studies in PD. There is still no exclusive biomarker to determine the expression of PD. Hence, it is convenient for complex clinic and genetic disorders such as behavioral ones to take into account the use of several sets of biomarkers. Thereby, it is necessary to include the assessment of groups of neurobiological, physiological and cognitive signals which allow to discern a specific phenotype. Also, each brain circuits shows complex constellations of behaviors that interact in the modulation of disorders. So far, the specific subtypes within PD are related to the orbitofrontal, dorsolateral and medial cingulate areas. However, neurobiological studies have yet not been able to locate a brain zone to explain the subtypes of PD. This condition is the result of an imbalance between stimulation and inhibition of different brain zones regarding a specific stimulus. In addition, current difficulties in approaching PD and their subtypes that constitute dimensions together with the differential expression of other comorbid syndromes and about which important clinical, neuropsychological, neurophysiological, biochemical and genetic differences are established (for example, age related with the severity of symptoms, the conduct disorder with or without child abuse and so on) (Evenden, 1999; Morgan & Lilienfeld, 2000; Sevecke, Lehmkuhl, & Krischer, 2008) suggest the necessity to advance in the taxonomy of theses syndromes through the use of several biomarkers that help to discriminate the subtype and establish a differential biological correlate. An exhaustive approximation to the spectrum of PD must include biomarkers that are co-related closely with impulsive subtypes, aggressive subtypes, and neurocognitive features associated with the patterns of behavior. These allow the distinction of the behavioral prototypes and the delineation of the genes that act in the expression of these patterns and in the interaction gene-environment, the epistatic phenomena and pleiotropism, as molecular phenomena that execute a potentially modulating effect of the clinical feature. Finally, this review aims to the development of phenomics, a concept recently introduced by Niculescu and Kelsoe in 2002 (Sabb et al., 2008), based on the characterization of phenotypes as a whole phenomenon (the phenotype and its interaction between genome and environment) that implies the recognition of critical features that delineate the clinical phenotype through multiple levels of intermediate traits. In fact, the psychiatric researchers are focusing around neurobiological and genetic traits linked with phenotype selection and specific clinical biomarkers and endophenotypes. Traits such as response inhibition, ERPs, emotional processing in PD are not unitary concepts; rather they are a potential construct in assessing specific tests. The reliability and validity of this fashion can be evaluated with different methods which enable inferences about the truth of the latent model (candidate biomarkers or putative endophenotypes). Therefore, understanding phenomics could also help to choose key phenotypes for psychiatric research.
8. FUTURE WORK
The study of endophenotypes and biomarkers in PD will provide strong empirical evidence which will help with the development of new elements to improve psychotherapies focused in specific alterations of the patients with these disorders. Traits associated with psychobiological or cognitive impairments will be understood and recognized by the health care system, psychiatrics and families.
Future research will include newer and more effective electrophysiological techniques available for neurocognitive, genetic, and clinical research. This tools will help to discover new genes associated with these disorders, new treatments as well as for a wide range of clinical applications.(Kamarajan & Porjesz, 2015) It is important to mention that the development of neuroimaging techniques have been enhanced in recent years, but their implement on the research of PD represents a recent issue.
Finally, a variety of new sophisticated statistical techniques could allow systematic research to highlight longitudinal aspects such as the cognitive development and the course of these disorders under research (Kamarajan & Porjesz, 2015).
9. REFERENCES
Allen, N. B., Lewinsohn, P. M., & Seeley, J. R. (1998). Prenatal and perinatal influences on risk for psychopathology in childhood and adolescence. Dev Psychopathol, 10(3), 513-529. [ Links ]
Amen, D. G., Stubblefield, M., Carmicheal, B., & Thisted, R. (1996). Brain SPECT findings and aggressiveness. Ann Clin Psychiatry, 8(3), 129-137. [ Links ]
Anderson, N. E., Stanford, M. S., Wan, L., & Young, K. A. (2011). High psychopathic trait females exhibit reduced startle potentiation and increased p3 amplitude. Behavioral sciences & the law, 29(5), 649-666. doi: 10.1002/bsl.998. [ Links ]
Anthenelli, R. M., Maxwell, R. A., Geracioti, T. D., Jr., & Hauger, R. (2001). Stress hormone dysregulation at rest and after serotonergic stimulation among alcohol-dependent men with extended abstinence and controls. [Clinical Trial Randomized Controlled Trial Research Support, U.S. Gov't, Non-P.H.S.Research Support, U.S. Gov't, P.H.S.]. Alcoholism, clinical and experimental research, 25(5), 692-703. [ Links ]
Bahmanyar, S., Montgomery, S. M., Weiss, R. J., & Ekbom, A. (2008). Maternal smoking during pregnancy, other prenatal and perinatal factors, and the risk of Legg-Calve-Perthes disease. Pediatrics, 122(2), e459-464. doi: peds.2008-0307 [pii] 10.1542/peds.2008-0307. [ Links ]
Bauer, L. O., & Hesselbrock, V. M. (2001). CSD/BEM localization of P300 sources in adolescents "at-risk": evidence of frontal cortex dysfunction in conduct disorder. Biol Psychiatry, 50(8), 600-608. doi: S0006322301010666 [pii] [ Links ]
Benjamin, J; Ebstein, R. P; Belmaker, R. H. (2001). Genes for Human Personality Traits: "Endophenotypes" of Psychiatric Disorders?. The World Journal of Biological Psychiatry, 2:2, 54-57. [ Links ]
Benning, S. D., Patrick, C. J., & Lacono, W. G. (2005). Psychopathy, startle blink modulation, and electrodermal reactivity in twin men. [Clinical Trial Research Support, N.I.H., Extramural Twin Study]. Psychophysiology, 42(6), 753-762. doi: 10.1111/j.1469-8986.2005.00353.x. [ Links ]
Berman, M. E; Coccaro, E. F. (1998). Neurobiologic correlates of violence: relevance to criminal responsibility. Behavioral Sciences & the Law, 16(3):303-18. [ Links ]
Birbaumer, N; Veit, R; Lotze, M; Erb, M; Hermann, C; Grodd, W; Flor, H. (2005). Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives of General Psychiatry, 62(7):799-805. [ Links ]
Blair, K. S., Richell, R. A., Mitchell, D. G., Leonard, A., Morton, J., & Blair, R. J. (2006). They know the words, but not the music: affective and semantic priming in individuals with psychopathy. [Research Support, N.I.H., Intramural]. Biological psychology, 73(2), 114-123. doi: 10.1016/j.biopsycho.2005.12.006. [ Links ]
Blanco, C., Alegria, A. A., Petry, N. M., Grant, J. E., Simpson, H. B., Liu, S. M., Hasin, D. S. (2010). Prevalence and correlates of fire-setting in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. The Journal of clinical psychiatry, 71(9), 1218-1225. doi: 10.4088/JCP.08m04812gry. [ Links ]
Boccardi, M., Frisoni, G. B., Hare, R. D., Cavedo, E., Najt, P., Pievani, M., . . . Tiihonen, J. (2011). Cortex and amygdala morphology in psychopathy. [Research Support, Non-U.S. Gov't]. Psychiatry research, 193(2), 85-92. doi: 10.1016/j.pscychresns.2010.12.013. [ Links ]
Boutwell, B. B., & Beaver, K. M. (2008). A biosocial explanation of delinquency abstention. Crim Behav Ment Health, 18(1), 59-74. doi: 10.1002/cbm.678. [ Links ]
Borod, Haywood & Koff. (1997). Neuropsychological aspects of facial asymmetry during emotional expressions: A review of the normal adult literature. Neuropsychology Review,7, 41 - 60. [ Links ]
Brennan PA, G. E. y. c. (1999). Maternal smoking during pregnancy and adult male criminal outcomes. Arch Gen Psychiatry,, 36, 215-219. [ Links ]
Brookes, K. J., Mill, J., Guindalini, C., Curran, S., Xu, X., Knight, J., Asherson, P. (2006). A common haplotype of the dopamine transporter gene associated with attention-deficit/hyperactivity disorder and interacting with maternal use of alcohol during pregnancy. [Research Support, Non-U.S. Gov't]. Archives of general psychiatry, 63(1), 74-81. doi: 10.1001/archpsyc.63.1.74. [ Links ]
Carmeli, C; Knyazeva, M. G; Cuenod, M; & Do, K. Q. (2012). Glutathione precursor Nacetyl-cysteine modulates EEG synchronization in schizophrenia patients: a double-Schiz 28 blind, randomized, placebo-controlled trial. PLoS One, 7(2), e29341. doi:10.1371/journal.pone.0029341. [ Links ]
Carlson, S. R., & Thai, S. (2010). ERPs on a continuous performance task and self-reported psychopathic traits: P3 and CNV augmentation are associated with Fearless Dominance. [Research Support, Non-U.S. Gov't]. Biological psychology, 85(2), 318-330. doi: 10.1016/j.biopsycho.2010.08.002. [ Links ]
Caspi, A; McClay, J; Moffitt, T E; Mill, J; Martin, J; Craig, IW; Taylor, A; Poulton, R. (2002). Role of genotype in the cycle of violence in maltreated children. Science, 297(5582):851-4. [ Links ]
Cuartas Arias, J. M., Palacio Acosta, C. A., Valencia, J. G., Montoya, G. J., Arango Viana, J. C., Nieto, O. C., Ruiz-Linares, A. (2011). Exploring epistasis in candidate genes for antisocial personality disorder. [Research Support, Non-U.S. Gov't]. Psychiatric genetics, 21(3), 115-124. doi: 10.1097/YPG.0b013e3283437175. [ Links ]
Coccaro, E. F; Kavoussi, R. J; Berman, M. E; Lish, J. D. (1998). Intermittent explosive disorder revised: Development, reliability, and validity of research criteria. Comprehensive Psychiatry, 39: 368 - 376. [ Links ]
Courtet, P; Gottesman, I; Jollant, F; Gould, T. D. (2011). The neuroscience of suicidal behaviors: what can we expect from endophenotype strategies?. Translational Psychiatry, 1(5): e7. doi: 10.1038/tp.2011.6. [ Links ]
Constantino, JN; Morris, JA; Murphy, DL. (1997). CSF 5-HIAA and family history of antisocial personality disorder in newborns. Journal of the American Psychiatric Association, 154(12):1771-3. [ Links ]
Daw, N. D., Kakade, S., & Dayan, P. (2002). Opponent interactions between serotonin and dopamine. Neural Netw, 15(4-6), 603-616. doi: S0893-6080(02)00052-7 [pii] [ Links ].
Diagnostic and Statistical Manual of Mental Disorders, fifth Edition. Arlington, VA, American Psychiatric Association, 2013. [ Links ]
DiLalla, L.F; Gottesman, II. (1991). Biological and genetic contributors to violence--Widom's untold tale. Psychological Bulletin, 109(1):125-129. [ Links ]
Dolan, M; Deakin, WJ; Roberts, N; Anderson, I. (2002). Serotonergic and cognitive impairment in impulsive aggressive personality disordered offenders: are there implications for treatment?. Psychological Medicine, 32(1):105-17. [ Links ]
Dolan, M; Fullam, R. (2006). Face affect recognition deficits in personality-disordered offenders: association with psychopathy. Psychological Medicine, 36(11):1563-1569. [ Links ]
Dongju Seo, C. J. P., Patrick J. Kennealy. (2008). Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggression and Violent Behavior., 13, 383-395. [ Links ]
Dougherty, D. D., Shin, L. M., Alpert, N. M., Pitman, R. K., Orr, S. P., Lasko, M., . . . Rauch, S. L. (1999). Anger in healthy men: a PET study using script-driven imagery. Biol Psychiatry, 46(4), 466-472. doi: S0006-3223(99)00063-3[pii] [ Links ].
Easton, C. J; Sacco, K. A; Neavins, T. M; Wupperman, P; George, T. P. (2008) Neurocognitive Performance Among Alcohol Dependent Men With and Without Physical Violence Toward Their Partners: A Preliminary Report', The American Journal of Drug and Alcohol Abuse, 34:1, 29 - 37. [ Links ]
Eling, P; Derckx, K; Maes, R. (2008). On the historical and conceptual background of the Wisconsin Card Sorting Test. Brain and Cognition,67(3):247-53. doi:10.1016/j.bandc.2008.01.006. [ Links ]
Evenden, J. L. (1999). Varieties of impulsivity. Psychopharmacology (Berl), 146(4), 348-361. doi: 91460348.213 [pii] [ Links ].
Fairbanks, L. A., Melega, W. P., Jorgensen, M. J., Kaplan, J. R., & McGuire, M. T. (2001). Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology, 24(4), 370-378. doi: S0893-133X(00)00211-6 [pii] 10.1016/S0893-133X(00)00211-6. [ Links ]
Fairchild, G., van Goozen, S. H., Stollery, S. J., Aitken, M. R., Savage, J., Moore, S. C., & Goodyer, I. M. (2009). Decision making and executive function in male adolescents with early-onset or adolescence-onset conduct disorder and control subjects. [Research Support, Non-U.S. Gov't]. Biological psychiatry, 66(2), 162-168. doi: 10.1016/j.biopsych.2009.02.024. [ Links ]
Fergusson, D. M., Boden, J. M., Horwood, L. J., Miller, A. L., & Kennedy, M. A. (2011). MAOA, abuse exposure and antisocial behaviour: 30-year longitudinal study. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. The British journal of psychiatry. the journal of mental science, 198(6), 457-463. doi:10.1192/bjp.bp.110.086991. [ Links ]
Fergusson, D. M., Woodward, L. J., & Horwood, L. J. (1998). Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch Gen Psychiatry, 55(8), 721-727. [ Links ]
Finger, E. C; Marsh, A. A; Mitchell, D. G. V; Reid, M. E; Sims, C; Budhani, S., et al. (2008). Abnormal ventromedial prefrontal cortex function in children with callous and unemotional traits during reversal learning. Archives of General Psychiatry, 65(5), 586 - 594. [ Links ]
Glenn, AL. (2010). The other allele: Exploring the long allele of the serotonin transporter gene as a potential risk factor for psychopathy: A review of the parallels in findings. Neuroscience & Biobehavioral Reviews, 35(3): 612-620. doi: 10.1016/j.neubiorev.2010.07.005. [ Links ]
Goldstein, R. B., & Grant, B. F. (2009). Three-year follow-up of syndromal antisocial behavior in adults: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't]. The Journal of clinical psychiatry, 70(9), 1237-1249. doi: 10.4088/JCP.08m04545. [ Links ]
Grant, B. F., Hasin, D. S., Stinson, F. S., Dawson, D. A., Chou, S. P., Ruan, W. J., & Pickering, R. P. (2004). Prevalence, correlates, and disability of personality disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry, 65(7), 948-958. [ Links ]
Hall, MH; Chen, CY; Cohen, BM; Spencer, KM; Levy,DL; Õngür, D; Smoller JW. (2015). Genomewide association analyses of electrophysiological endophenotypes for schizophrenia and psychotic bipolar disorders: a preliminary report. American Journal of Medical Genetics, 168B(3):151-61. doi: 10.1002/ajmg.b.32298. [ Links ]
Hare, R. D. (1982). Psychopathy and physiological activity during anticipation of an adversive stimulus in a distraction paradigm. Psychophysiology, 19(3), 266-271. [ Links ]
Hare, R. D., Clark, D., Grann, M., & Thornton, D. (2000). Psychopathy and the predictive validity of the PCL-R: an international perspective. Behav Sci Law, 18(5), 623-645. doi: 10.1002/1099-0798(200010)18:5<623::AID-BSL409>3.0.CO;2-W [pii] [ Links ].
Hare, R. D., & Quinn, M. J. (1971). Psychopathy and autonomic conditioning. J Abnorm Psychol, 77(3), 223-235. [ Links ]
Hecht, D. (2010). Psychopathy and fearlessness: an interhemispheric imbalance perspective. [Comment Letter]. Biological psychiatry, 67(8), e51-52. doi: 10.1016/j.biopsych.2009.10.034. [ Links ]
Herpertz, S. C; Huebner, T; Marx, I; Vloet, T. D; Fink, G. R; Stoecker, T; Shah, N. J; Konrad, K; Herpertz-Dahlmann, B. (2008) Emotional processing in male adolescents with childhood-onset conduct disorder. Journal of Child Psychology and Psychiatry, 49(7):781-91. doi: 10.1111/j.1469-7610.2008.01905.x. [ Links ]
Herpertz, S. C., Werth, U., Lukas, G., Qunaibi, M., Schuerkens, A., Kunert, H. J., Sass, H. (2001). Emotion in criminal offenders with psychopathy and borderline personality disorder. Arch Gen Psychiatry, 58(8), 737-745. doi: yoa20015 [pii] [ Links ].
Hiatt, K. D; Schmitt, W. A; Newman, J. P. (2004). Stroop Tasks Reveal Abnormal Selective Attention Among Psychopathic Offenders. Neuropsychology, 18(1): 50 - 59. doi: : 10.1037/0894-4105.18.1.50. [ Links ]
Hoptman, M. J. (2003). Neuroimaging studies of violence and antisocial behavior. J Psychiatr Pract, 9(4), 265-278. doi: 00131746200307000-00002 [pii] [ Links ].
Huchzermeier, C., Geiger, F., Kohler, D., Bruss, E., Godt, N., Hinrichs, G., & Aldenhoff, J. B. (2008). Are there age-related effects in antisocial personality disorders and psychopathy? J Forensic Leg Med, 15(4), 213-218. doi: S1752-928X(07)00153-9 [pii] 10.1016/j.jflm.2007.10.002. [ Links ]
Huebner, T., Vloet, T. D., Marx, I., Konrad, K., Fink, G. R., Herpertz, S. C., & Herpertz-Dahlmann, B. (2008). Morphometric brain abnormalities in boys with conduct disorder. [Research Support, Non-U.S. Gov't]. Journal of the American Academy of Child and Adolescent Psychiatry, 47(5), 540-547. doi: 10.1097/CHI.0b013e3181676545. [ Links ]
Kamarajan, C; Porjesz, B. (2015). Advances in Electrophysiological Research. Current Reviews, 37(1): 53 - 87. [ Links ]
Kosson DS1, Lorenz AR, Newman JP. (2006). Effects of comorbid psychopathy on criminal offending and emotion processing in male offenders with antisocial personality disorder. Journal of Abnormal Psychology 115(4):798-806. [ Links ]
Kostandov, E. A., Tal'tse, M. F., Zakharova, N. N., & Vazhnova, T. N. (1994). [The psychophysiological criteria for assessing the excitable type of psychopathy]. Zh Nevrol Psikhiatr Im S S Korsakova, 94(5), 64-71. [ Links ]
Lang, P. J., Greenwald, M. K., Bradley, M. M., & Hamm, A. O. (1993). Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology, 30(3), 261-273. [ Links ]
Lobbestael, J., Arntz, A., Cima, M., & Chakhssi, F. (2009). Effects of induced anger in patients with antisocial personality disorder. Psychological medicine, 39(4), 557-568. doi: 10.1017/S0033291708005102. [ Links ]
Malterer, M. B; Glass, S. J; Newman, J. P. (2008). Psychopathy and Trait Emotional Intelligence. Personality and Individual Differences, 44(3): 735-745. doi: 10.1016/j.paid.2007.10.007. [ Links ]
Marsh, A. A., & Blair, R. J. (2008). Deficits in facial affect recognition among antisocial populations: a meta-analysis. [Meta-Analysis Research Support, N.I.H., Intramural Review]. Neuroscience and biobehavioral reviews, 32(3), 454-465. doi:10.1016/j.neubiorev.2007.08.003. [ Links ]
Mehlman, P. T., Higley, J. D., Faucher, I., Lilly, A. A., Taub, D. M., Vickers, J., .Linnoila, M. (1994). Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry, 151(10), 1485-1491. [ Links ]
Meijer, E. H., Smulders, F. T., Johnston, J. E., & Merckelbach, H. L. (2007). Combining skin conductance and forced choice in the detection of concealed information. Psychophysiology, 44(5), 814-822. doi: 10.1111/j.1469-8986.2007.00543.x. [ Links ]
Moller, S. E., Mortensen, E. L., Breum, L., Alling, C., Larsen, O. G., Boge-Rasmussen, T., ... Bennicke, K. (1996). Aggression and personality: association with amino acids and monoamine metabolites. Psychol Med, 26(2) [ Links ]
Morera, A; Gonzalez, P; Henry, M. (2009). Zaleplon increases nocturnal melatonin secretion in humans. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 31;33(6):1013-6. doi: 10.1016/j.pnpbp.2009.05.011. [ Links ]
Muller, J. L., Ganssbauer, S., Sommer, M., Dohnel, K., Weber, T., Schmidt-Wilcke, T., & Hajak, G. (2008). Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel-based morphometry. Psychiatry research, 163(3), 213-222. doi: 10.1016/j.pscychresns.2007.08.010. [ Links ]
Narayan, V. M., Narr, K. L., Kumari, V., Woods, R. P., Thompson, P. M., Toga, A. W., & Sharma, T. (2007). Regional cortical thinning in subjects with violent antisocial personality disorder or schizophrenia. Am J Psychiatry, 164(9), 1418-1427. doi: 164/9/1418 [pii] 10.1176/appi.ajp.2007.06101631. [ Links ]
Nelson, R. J., & Chiavegatto, S. (2001). Molecular basis of aggression. Trends Neurosci, 24(12), 713719. doi: S0166-2236(00)01996-2 [pii] [ Links ].
Onyekwere, D; Ramírez, J. M. (1994). Influence of timing of post-weaning isolation on play fighting and serious aggression in the male golden hamster (Mesocricetus auratus). Aggressive Behavior, 20(2):115-122. DOI: 10.1002/1098-2337(1994)20:23.0.CO;2-J. [ Links ]
Ortony, A., & Turner, T. J. (1990). What's basic about basic emotions? Psychological Review, 97,315-331. [ Links ]
Ostrosky, F., Gómez, E., Matute, E., Rosselli, M., Ardila, A., & Pineda, D. (2007). NEUROPSI ATTENTION AND MEMORY: Aneuropsychological test battery in Spanish with norms by age and educational level. Applied Neuropsychology, 14(3), 156-170. [ Links ]
Pajer, K; Gardner, W; Rubin, R T; Perel, J; Neal, S. (2001). Decreased cortisol levels in adolescent girls with conduct disorder. Archives of General Psychiatry, 58(3):297-302. [ Links ]
Panksepp. (1992). Handbook of Communication and Emotion: Research, Theory, Applications, and Contexts. London: Peter & Laura. [ Links ]
Patrick, C. J., Bernat, E. M., Malone, S. M., Iacono, W. G., Krueger, R. F., & McGue, M. (2006). P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology, 43(1), 84-92. doi: PSYP376 [pii] 10.1111/j.1469-8986.2006.00376.x. [ Links ]
Philibert, R. A., Wernett, P., Plume, J., Packer, H., Brody, G. H., & Beach, S. R. (2011). Gene environment interactions with a novel variable Monoamine Oxidase A transcriptional enhancer are associated with antisocial personality disorder. [Research Support, N.I.H., Extramural]. Biological psychology, 87(3), 366-371. doi: 10.1016/j.biopsycho.2011.04.007. [ Links ]
Prehn, K., Schulze, L., Rossmann, S., Berger, C., Vohs, K., Fleischer, M., ... Herpertz, S. C. (2012). Effects of emotional stimuli on working memory processes in male criminal offenders with borderline and antisocial personality disorder. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. doi: 10.3109/15622975.2011.584906. [ Links ]
Racer, K. H., Gilbert, T. T., Luu, P., Felver-Gant, J., Abdullaev, Y., & Dishion, T. J. (2011). Attention network performance and psychopathic symptoms in early adolescence: an ERP study. [Research Support, N.I.H., Extramural]. Journal of abnormal child psychology, 39(7). 1001-1012. doi: 10.1007/s10802-011-9522-6. [ Links ]
Raine, A., Brennan, P., & Mednick, S. A. (1997). Interaction between birth complications and early maternal rejection in predisposing individuals to adult violence: specificity to serious, early-onset violence. Am J Psychiatry, 154(9), 1265-1271. [ Links ]
Ramirez, J. M., & Andreu, J. M. (2006). Aggression, and some related psychological constructs (Anger, hostility, and impulsivity. Some comments from a research project). Neuroscience and Biobehavioral Reviews, 30, 276-291. [ Links ]
Ramirez OA, Carrer HF. (1982) Effect of estrogen and progesterone priming on the uptake and release of serotonin and noradrenaline from the ventromedial hypothalamus. Acta Physiol Lat Am. 32, 313-319. [ Links ]
Ramirez, J. M. and Delius, J. D. (1979), Aggressive behavior of pigeons: Suppression by archistriatal lesions. Aggr. Behav., 5: 3-17. doi: 10.1002/1098-2337(1979)5:1<3::AID-AB2480050103>3.0.CO;2-5. [ Links ]
Ritsner, M.S; Gottesman, II. (2009). Where Do We Stand in the Quest for Neuropsychiatric Biomarkers and Endophenotypes and What Next?. In Michael S & Ritsner M. D. (Ed.) The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes (pp. 3 -21). [ Links ]
Sadeh, N; Verona, E. (2008) Psychopathic traits associated with abnormal selective attention and impaired cognitive control. Neuropsychology.22:669-680. [ Links ]
Sabb, F. W., Bearden, C. E., Glahn, D. C., Parker, D. S., Freimer, N., & Bilder, R. M. (2008). A collaborative knowledge base for cognitive phenomics. Mol Psychiatry, 13(4), 350-360. doi: 4002124 [pii] 310.1038/sj.mp.4002124. [ Links ]
Samuels, J. (2011). Personlaity Disorders: epidemiology and public health issues. Int Rev Psychiatry, 23(3): 223 – 233. doi 10.3109/09540261.2011.588200. [ Links ]
Schalling, D., Asberg, M., Edman, G., & Oreland, L. (1987). Markers for vulnerability to psychopathology: temperament traits associated with platelet MAO activity. Acta Psychiatr Scand, 76(2), 172-182. [ Links ]
Sevecke, K., Lehmkuhl, G., & Krischer, M. K. (2008). Examining relations between psychopathology and psychopathy dimensions among adolescent female and male offenders. Eur Child Adolesc Psychiatry. doi: 10.1007/s00787-008-0707-7. [ Links ]
Sher, L; Oquendo, M A; Galfalvy, H C; Grunebaum, M F; Burke, A K; Zalsman, G; Mann, J J. (2005). The relationship of aggression to suicidal behavior in depressed patients with a history of alcoholism. Addictive Behaviors, 30: 1144 – 1153. [ Links ]
Siever, LJ. (2005). Endophenotypes in the personality disorders. Dialogues Clin Neurosci, 7(2): 139 – 151. [ Links ]
Soderstrom, H., Hultin, L., Tullberg, M., Wikkelso, C., Ekholm, S., & Forsman, A. (2002). Reduced frontotemporal perfusion in psychopathic personality. Psychiatry Res, 114(2), 81-94. doi: S0925492702000069 [pii] [ Links ].
Stevens, M. C., Kaplan, R. F., & Hesselbrock, V. M. (2003). Executive-cognitive functioning in the development of antisocial personality disorder. Addict Behav, 28(2), 285-300. doi: S0306460301002325 [pii] [ Links ].
Sundram, F., Deeley, Q., Sarkar, S., Daly, E., Latham, R., Craig, M., Murphy, D. G. (2012). White matter microstructural abnormalities in the frontal lobe of adults with antisocial personality disorder. [Research Support, Non-U.S. Gov't]. Cortex; a journal devoted to the study of the nervous system and behavior, 48(2), 216-229. doi: 10.1016/j.cortex.2011.06.005. [ Links ]
Taylor, J., Loney, B. R., Bobadilla, L., Iacono, W. G., & McGue, M. (2003). Genetic and environmental influences on psychopathy trait dimensions in a community sample of male twins. J Abnorm Child Psychol, 31(6), 633-645. [ Links ]
Terburg, D., Morgan, B., & van Honk, J. (2009). The testosterone-cortisol ratio: A hormonal marker for proneness to social aggression. [Review]. International journal of law and psychiatry, 32(4), 216-223. doi: 10.1016/j.ijlp.2009.04.008. [ Links ]
Virkkunen, M., Ebeling, H., Moilanen, I., Tani, P., Pennanen, S., Liesivuori, J., & Tiihonen, J. (2003). Total plasma l-tryptophan, free l-tryptophan and competing amino acid levels in a homicidal male adolescent with conduct disorder. [Case Reports]. Acta psychiatrica Scandinavica, 108(3), 244-246; discussion 246-247. [ Links ]
Wermter, A. K., Laucht, M., Schimmelmann, B. G., Banaschweski, T., Sonuga-Barke, E. J., Rietschel, M., & Becker, K. (2010). From nature versus nurture, via nature and nurture, to gene x environment interaction in mental disorders. [Review]. European child & adolescent psychiatry, 19(3), 199-210. doi: 10.1007/s00787-009-0082-z. [ Links ]
World Health Organization. (2005). ICD-10 international statistical classification of diseases and related health problems (pp. 1 CD-ROM 4 3/4 in. [ Links ]).