Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
International Journal of Psychological Research
Print version ISSN 2011-2084
int.j.psychol.res. vol.9 no.2 Medellín Jul./Dec. 2016
https://doi.org/10.21500/20112084.2303
DOI: http://dx.doi.org/10.21500/20112084.2303
Research
Variation in glucocorticoid levels: survival and reproductive demands in wild black capuchins (Sapajus nigritus)
Variación en los niveles de glucocorticoides: demandas de supervivencia y reproducción en capuchinos negros salvajes (Sapajus nigritus)
Caio M. Moreiraa*, Lucas Peternelli dos Santosa, Maria Bernardete C. de Sousab and Patrícia Izara
a Department of Experimental Psychology, University of São Paulo, Brazil.b Brain Institute, Federal University of Rio Grande do Norte, Brazil.
* Corresponding author: Caio Moreira, Department of Experimental Psychology, University of São Paulo, Av. Prof. Mello Moraes 1721, São Paulo - 05508-030, São Paulo, Brazil. Email address: caiommoreira@gmail.com.
Article history:
Received: 14-04-2016. Revised: 20-06-2016. Accepted: 29-06-2016.
ABSTRACT
According to the concept of allostasis and its association with energy mobilization, glucocorticoids (GCs) should parallel cumulative energy expenditure for animal survival and reproduction. Therefore, it is expected that seasonal food shortages might lead to increased levels of GCs. We tested this hypothesis by analyzing the intra-annual variation of fecal glucocorticoid metabolites (GCM) in 14 wild black capuchin monkeys (Sapajus nigritus) living in a social group in the Brazilian Atlantic Forest. We analyzed the association between GCM and social and environmental variables for the three age/sex classes (adult males, adult females and immatures (juveniles and infants). Decreased fruit intake during the dry season increased the GCM levels of both immatures and adult males. Although fruit shortage influenced the allostasis of adult males, the variation in their GCM levels was more impacted by the breeding season. GCM levels of adult females varied during the late stage of pregnancy. These results suggest fruit consumption as the main source of allostatic load for immature animals, while reproductive costs had greater effect on adults.
Key words: fecal glucocorticoids, capuchin monkey, Sapajus, tropical forest, development, reproductive behavior.
RESUMEN
De acuerdo al concepto de alostasis y su asociación con la movilización de la energía, los glucocorticoides (GC) deben acumular en paralelo la energía para la supervivencia animal y la reproducción. Por lo tanto, se espera que la escaséz de alimentos de temporada pueda llevar al incremento de los niveles de GC. Pusimos a prueba esta hipótesis mediante el análisis de la variación intra- anual de metabolitos de glucocorticoides fecales (MGC) en 14 monos capuchinos salvajes negros (Sapajus nigritus), que vivían en un grupo social en el bosque Atlántico de Brasil. Analizamos la asociación entre MGC y variables sociales y ambientales para tres clases de edad y sexo (machos adultos, hembras adultas y jóvenes e infantes inmaduros). La disminución en la ingesta de fruta durante la temporada seca, incrementó los niveles de MGC en los machos inmaduros y adultos. A pesar de que la escasez de fruta influyó en la alostasis de los adultos machos, la variación en sus niveles de MGC fueron mas impactados en la temporada de reproducción. Los niveles MGC en hembras adultas variaron durante la última etapa del embarazo. Estos resultados sugieren el consumo de frutas como principal fuente de carga alostática para animales inmaduros, mientras que los costos reproductivos tienen mayor efecto para los adultos.
Palabras clave: glucocorticoides fecales, mono capuchino, Sapajus, bosque tropical.
1. INTRODUCTION
Stressors are usually defined as stimuli that disrupt the body's homeostasis - i.e. stability of physiological parameters (Chrousos & Gold, 1992). Nevertheless, studies that estimate long-term effects of stressors have been focusing on allostasis - i.e. the ability of the body to produce mediators that support the animal adaptation to a new situation - to explain how the body changes in response to stressor events (McEwen, 1998). Allostasis is not only related to acute environmental disturbances, but also to the body's ability to deal with predicted events occurring at different intervals of the day and on different seasons or periods of the life cycle (McEwen & Wingfield, 2003; McEwen & Wingfield, 2010; Romero, Dickens, & Cyr, 2009).
In this context, energy is intrinsically related to allostasis because it influences the physiological mechanisms needed to adjust internal demands to environmental changes. If the energy required by the organisms to deal with their routine or with unpredictable events (allostatic load) exceeds the energy they can acquire from the environment, the levels of hormones associated with energy mobilization, such as glucocorticoids (GCs), usually raise to initiate physiological and behavioral changes that promote survival (Sapolsky, Romero, & Munck, 2000; McEwen & Wingfield, 2007; Wingfield & Ramenofsky, 1999). That is why energy intake shortages have been associated with high levels of GCs in several primate species (Alouatta pigra: Behie, Pavelka, & Chapman, 2010; Lemur catta: Cavigelli, 1999; Pan troglodytes: Muller & Wrangham, 2004; Papio anubis: Sapolsky, 1986; Papio ursinus: Weingrill, Gray, Barrett, & Henzi, 2004) and correlated with travel distance (Di Fiore & Suarez, 2007; Girard & Garland, 2002).
Previous studies suggested that the black capuchin monkeys (Sapajus nigritus) living at Carlos Botelho State Park (PECB) - an area of Atlantic Forest in São Paulo state, Brazil - present low reproduction rate, large home range and foraging in subgroups as a consequence of nonideal environmental condition characterized by low food availability, especially during the dry season (Izar, 2004; Izar, Stone, Carnegie, & Nakai, 2009; Izar et al., 2012). In this context, and using the association between energy mobilization and GCs, we verified whether energy shortage during the dry season would result in higher levels of fecal glucocorticoid metabolites (GCM) on individuals' feces.
Since adult animals have specific reproduction-related requirements also known to influence the production of GCs, we investigated whether there was an increase in males' GCM levels during the breeding season in response to mating opportunities or intragroup male-male competition (e.g. Lynch, Ziegler, & Strier, 2002; Romero et al., 2009; Sapolsky, 1983; Setchell, Smith, Wickings, & Knapp, 2010; Strier, Ziegler, & Wittwer, 1999). We also investigated whether there is an increase in females' GCM levels caused by energy requirements related to the breeding season or to the late pregnancy (Bales, French, Hostetler, & Dietz, 2005; Bercovitch & Ziegler, 2002; Lahoz, Nagle, & Porta, 2007; Schoof, Jack, & Ziegler, 2014); and if reproduction-related factors can explain GCM variations better than food availability. Since immature individuals do not have reproduction-related requirements and deal with specific challenges associated to their development, as body growth (Altmann, 1998; Clymer, 2006), we expected that food availability would influence their GCM levels more than other events. We therefore predicted that immature individuals would differ in their GC responses from adults and that adult males would differ from adult females.
2. METHODS
2.1 Study area
Data collection took place in PECB, located in southeastern Brazil in an Atlantic Forest domain of São Paulo state (24º00'-24º15'S, 47º45'-48º10'W). It comprises an area of about 380 km2, and forms, with three other parks, a continuous and mainly undisturbed forest within an area of 1200 km2 (Dias, Custodio Filho, Franco, & Couto, 1995). The study site is located at an altitude of 720-890 m and has average annual temperatures between 19oC and 22ºC, with a minimum of 3oC and maximum of 29oC. High rainfall levels occur between September and March (spring and summer) and the lowest level occurs in July during the winter (Presotto & Izar, 2010; Izar et al., 2012). The winter season (from June until September) is also the period of the year when fruit availability reaches its lowest levels (Izar, 2004; Izar et al., 2012).
2.2 Group composition
At the end of the study period, the monitored group of S. nigritus, denominated Pimenta 1, had 14 individuals: 2 adult males, 3 adult females, 7 juveniles and 2 infants. The group did not vary during this period, except for one birth in September/October 2007. We were able to classify the individuals into age/sex classes (adult males, adult females and immatures) because all adult members were individually recognized based on previous studies of the group (Izar, 2004; Nakai, 2007; Taira, 2007), and based on color patterns, body size and cap shape.
2.3 Sampling
The group Pimenta 1 was monitored during 3-8 days per month (mostly between sunrise and sunset) from August 2007 to July 2008. The number of hours of group observation was 103, 89, 98, 42, 46, 0, 42, 23, 72, 48, 58 and 34 per month, respectively over the 12-month study period. The group had been habituated to the presence of humans for three years.
2.3.1 Behavior sampling
In order to access individuals' behavior, we recorded 1 minute scan sampling of all individuals we could observe followed by 4 minutes without recordings (Altmann, 1974) during the entire observation period. Animals' activities were categorized as: foraging, resting, traveling or social behaviors as in Izar et al. (2012). We also performed ad libitum sampling of female proceptivity, sexual interactions and births. Female proceptive behavior is described by Izar (2009). Group location was recorded every 5 minutes, at the beginning of each scan sampling, using a Map 60 CSx GPS handset as described by Presotto & Izar (2010).
2.3.2 Glucocorticoid metabolites sampling
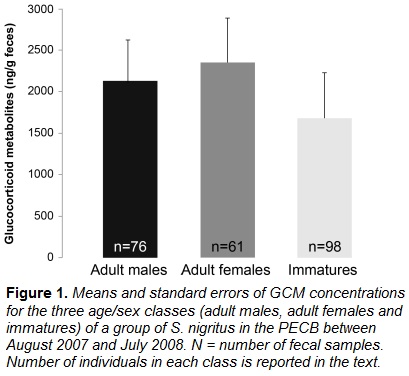
Fecal samples were collected from all age/sex classes in the group on an opportunistic basis in order to gather the maximum number of samples. We collected only fresh fecal samples in which the individual (adults) or the individual's age class (immatures) was identified. The total number of samples per age/sex class was: 76 for adult males, 61 for adult females and 98 for immatures. The time of collection was recorded and the results were pooled taking into account the time of day: from 6:00h to 10:00h; from 10:01h to 14:00h; and from 14:01h to 18:00h, for further considerations about circadian rhythm. After collection using a hood stick, the fresh samples were placed in 15 ml falcon tubes and kept on ice until being transferred to the facility where remained at -20°C until the analysis.
2.4 Data sorting
Animals' activity budget encompasses the percentage of time they spent in foraging, resting, traveling and social behaviors. The diet was accessed by dividing foraging into proportions of time animals invested in: fruits, invertebrates or leaves. Since the diet of capuchin monkeys at PECB consists mainly of fruits, invertebrates and epiphytic bromeliad leaves, and only fruits exhibit limited availability (Izar et al., 2012), we used the proportion of fruit consumption in relation to total foraging scans of each age/sex class as a measure of food availability.
Since we expected that the breeding season influences the variation of GCMs of adult males, and late pregnancy influences the variation of GCMs of adult females, the whole study period was divided into smaller reproduction-related periods based on former studies (Izar et al., 2009), on our personal observations of sexual activity and based on birth records. These periods are described on Data analysis. Birth records were determined during the study period and through follow-up censuses of the group between September and November 2008. We recorded only one female displaying proceptive behavior followed by copulation on April 2008, but two females gave birth 6 months after this period.
2.5 Steroid processing
Fecal samples were processed according to methods reported by Sousa & Ziegler (1998). Briefly, 0.1 g of feces was weighed and extracted into 5 ml of ethanol/water. A 500 µl portion was taken for solvolysis (Ziegler et al., 1996) and samples were resuspended in 500 µl of ethanol and stored until assay quantification. GC metabolite assays, being cortisol the main hormone (60%), were performed using the fecal extracts. The flat-bottomed microtiter plates (Nunc) used to perform ELISA were previously sensitized with polyclonal anticortisol R4866. The enzyme conjugated to the antigen was horseradish peroxidase. Both antibody (R4866) and HRP (Cortisol:HRP) were prepared by C. Munro (University of California, Davis, CA, USA), used at respective dilutions of 1:16.000 and 1:75.000. The standards ranged from 3.16 to 1000 pg/ml. The validation protocol for GCM in feces was developed in the Laboratory for Hormonal Measurement at Universidade Federal do Rio Grande do Norte, Brazil. The intra- and inter-assay variation coefficients for low and high pools were 1.61 ± 0.19 % and 3.47 ± 2.47 %, and 6.6 ± 5.19 % to 10.84 ± 0.72 %, respectively.
3. DATA ANALYSIS
ArcView v9.3 was used to calculate monthly travelled distances using plotted location points. These distances were incorporated into the model as described below.
We used one single model to test the effect of the independent factors (day period, monthly travelled distance, food availability, breeding season and late pregnancy) on GCM levels. We performed the analysis in R software using a linear mixed-effects regression model (R package nlme; Pinheiro et al., 2014) that allowed us to include all data available (unbalanced design) with GCM levels as response. We included the identity of each adult animal as random effect. Juveniles and infants were also included as random effect variables but, since we could not distinguish the individuals, they were included as two individual variables. Significance for statistical tests was established as p < 0.05.
Since we had specific predictions for each age/sex class about the influence of food availability and the breeding season over individuals' GCM levels, we incorporated the interaction between these variables and age/sex classes. Food availability corresponded to the monthly percentage of fruit consumption in the diet of each age/sex class; and the breeding season was modeled as factor. Considering the recorded sexual activity, the typical gestation period for S.nigritus (five months) and follow-up birth censuses, we defined the breeding season during April (see also Izar et al., 2009). We also incorporated time of the day and monthly travelled distance as variables without interaction, since we did not expect age/sex differences. Late pregnancy was modeled as factor for the only adult female who was at this stage of pregnancy during the study period.
Hence, the used linear mixed-effects regression model (lme) was the following:
Model = lme (GCM ~ Day period + Fruit availability*Age/sex class + Breeding season*Age/sex class + Pregnancy + Distance, random = ~ 1 Individual)
4. RESULTS
No significant differences in mean GCM levels were found among age/sex classes (DF=4, p > 0.05 for all comparisons), but adult females had the highest mean GCM levels, followed by adult males, and immatures (Figure 1).
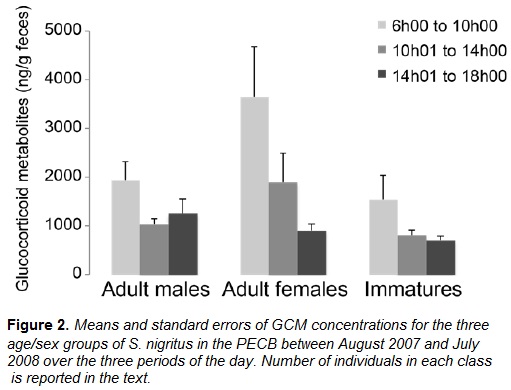
The group's GCM levels decreased after the first period of the day. Compared to interval one (6-10am), GCM levels decreased significantly in interval two (t(216) = -4.193, p < 0.0001) and three (t(216) = -5.218, p < 0.0001). No difference between the second and third intervals of the day was observed (t(216) = -1.383, p = 0.17). The decrease on GCM levels after the first period of the day occurred for the three age/sex classes (Figure 2).
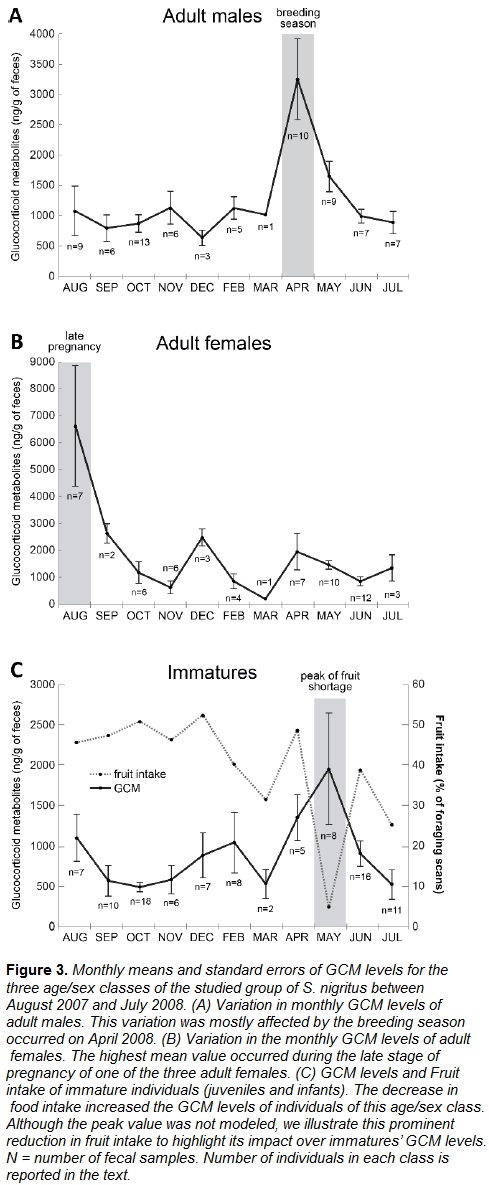
Food availability and reproduction-related factors affected differently the GCM levels of each age/sex class. A decrease in 1% of food availability resulted in an increase of 25.11±11.12 ng of GCM/g of feces in immatures (t(216) = -2.636, p < 0.01) and 14.19±7.19 ng of GCM/g of feces in adult males (t(216) = -1.975, p < 0.05), but it did not influence adult females' GCM levels (t(216) = -0.001, p = 0.99).
During the breeding season (April), the GCM levels of adult males increased 2225.14±378.35 ng/g of feces (t(216) = 5.869, p < 0.0001), while it increased only 798.80±590.01 ng/g of feces in adult females (t(216) = 1.753, p = 0.08) and 494.47±634.19 ng/g of feces in immatures (t(216) = 1.415, p = 0.16). For the last two age/sex classes, the variation was not statistically significant.
Lastly, the only adult female who was on late pregancy during the study period presented fecal GCM levels higher at this stage than during other periods (t(216) = 13.906, p < 0.0001).
We highlighted the modeled variables that influenced most the variation of GCM levels of each of the age/sex classes: food availability for immatures, breeding season for adult males and late pregnancy for adult females, using GCM monthly means (Fig. 3).
Travelled distance did not influence GCM levels (t(216) = 0.583, p = 0.56).
5. DISCUSSION
The data sampling of the present study was restricted by the small size of the studied group of S. nigritus living in PECB (especially the low number of adult individuals) compared to other populations (e.g. Lynch & Rímoli, 2000). Despite this intrinsic limitation, we showed that food shortages affected the GCM levels of adult males and especially of immature individuals. This result agrees with previous studies relating peculiarities of this population - low reproduction rate, large home range and foraging in subgroups - to seasonal decreases in food availability (Izar, 2004; Izar et al., 2009; Izar et al., 2012).
In order to deal with our restricted sample size, we used a sensitive approach in which one single model tested the interactions of all modelled factors and controlled for repeated sampling of the same individuals. We found results regarding GCM diurnal variation similar to those obtained by field and laboratory studies (e.g Kriegsfeld & Silver, 2006; Torres-Farfan et al., 2008) and we obtained significant results about the influence of different factors over individuals' GCM levels depending on their age/sex classes. Specifically, the variation of GCM levels of immature animals was associated with decreased fruit intake; the GCM levels of adult males increased mostly during the breeding season; and the variation of GCM levels in adult females might be explained by the increased energy demand during the late stage of pregnancy.
Food availability was the main seasonal factor influencing the GCM levels of immature capuchins. Immature individuals exhibit higher metabolic rates (Bell, 1971; Kleiber, 1961) and have additional energy costs for growth (Altman, 1998). These costs should be balanced with a proportionally higher energy intake (Agetsuma, 2001; Clymer, 2006; Oftedal, 1991). In general, fruits are high quality resources because they are easy to digest and rich in energy (Garber, 1998; Chapman et al., 2012), playing an important role in capuchins' diet (Izar et al., 2012). Fruits are, therefore, essential to increase the energy intake of juveniles and infants. Moreover, a concurrent study performed with the same capuchin group showed that fruit shortage has more detrimental effects on juveniles because these animals are the main targets of agonistic behavior in foraging-related contexts (Peternelli-dos-Santos, 2010). Consequently, juveniles foraged more on non-patchily distributed resources such as leaves, than on monopolizable foods such as fruits. According to the present data, fruit foraging accounted for only 4.65% of the feeding time (foraging) of juveniles and infants during the peak of fruit shortage (May), and close to 40% during summer (from December until March). Based on our data and on the association between allostasis and energy mobilization (McEwen & Wingfield, 2007), we propose that immature capuchins might use GCs to mobilize energy to search, extract and process low quality foods. Bromeliad leaves, for instance, were consumed during 70% of the foraging time on the peak of fruit consumption shortage (May).
As previously described, whereas GCM levels of adult males were influenced by fruit intake, they were mostly influenced by the mating season. Cortisol levels of male S. nigritus are shown to increase during this season in response to mating opportunities (Lynch et al. 2002). Similar results were also reported for Cebus capucinus (Schoof et al., 2014), Brachyteles arachnoides (Strier et al., 1999) and Callithrix jacchus (Cunha, Vivacqua, Fernandes, & Sousa, 2007). Although the variation in GCs of primate males was also associated with intragroup male-male competition (Emery Thompson & Georgiev, 2014), we did not find such association in our data (Moreira, 2010). The studied group of capuchin monkeys had only two adult males, being the beta male mostly positioned at the periphery of the group with rare interactions with other adult individuals. Nevertheless, since the GCM levels of dominant males of Sapajus libidinosus increase due to energetic costs related to group protection against predators and interventions on intragroup conflicts (Mendonça-Furtado et al., 2014), it is possible that these or similar factors were adding extra burden to the allostatic load that the PECB's male capuchins had during the breeding season.
Finally, the variation of adult females' GCM levels was not associated with fruit intake or with the breeding season. Their highest GCM values occurred during the late pregnancy period of one female. The increase in GCM during this pregnancy stage is expected since, in many primate species, baseline cortisol levels rise during the latest stage of gestation to boost fetus's brain development and lung maturation (e.g. human: Lindsay & Nieman, 2005; mandrill: Setchell, Smith, Wickings, & Knapp, 2008; common marmoset: Tardif, Ziegler, Power, & Layne, 2005; cotton-top tamarin: Ziegler, Scheffler, & Snowdon, 1995). High cortisol levels are also associated with lactation in chimpanzees (Emery Thompson, Muller, Kahlenberg, & Wrangham, 2010), but the present data suggest that this is not the case for S. nigritus, since GCM levels dropped after parturition. Our results are, therefore, similar to those found by Ganzhorn (2002) suggesting that reproduction in adult (male and female) lemurs imposes higher costs than decreased food availability. Nevertheless, considering that the concentration of GCM on females' feces during the late pregnancy was much higher than during the following months (or even compared to the peak levels of the other two age/sex classes), we ran another model without the data related to this period in order to test if fruit intake and/or the breeding season influenced the allostasis of females, but their effects were masked by the greater variation of GCM levels on August. Fruit intake and breeding season also did not influence the GCM levels of adult females in this additional model (data not presented), indicating that the proceptive behavior exhibited by females prior to the copulation did not affect their GCM levels, or we were not able to collect samples which contained evidence about the physiological changes associated to this behavioral demonstration. Therefore, our results suggest that in spite of low fruit availability, females had access to the energy required by their allostatic load during the studied period.
In summary, in spite of the limitations of our study due to small sampling, we provided evidence that the physiology of S. nigritus living in an area of Atlantic Forest at southeastern Brazil is modified to deal with annual variation in fruit availability. In this context, low fruit availability seems to be critical for animals in development (juveniles and infants), but the reproduction is still the major demand for adults.
6. ACKNOWLEDGMENTS
We thank Eraldo Vieira, our field assistant, and the students Lucas Peternelli and Mariana Dutra Fogaça for help with field data collection. We thank the Instituto Florestal de São Paulo, specially the PECB manager, José Carlos Maia, for permission to conduct this research, and the Brain Institute of The Federal University of Rio Grande do Norte for the support for hormonal analysis. We thank an anonymous reviewer for helpful comments. We also thank the reviewers of the International Journal of Primatology which provided essential comments on an earlier version of the manuscript, modifying our data analysis, and Dr. Holger Sennhenn-Reulen for the statistical support. This research was supported by FAPESP grant 06/56059-0 to P.I, FAPESP grant 07/57000-1 to C.M and CNPq grants to P.I. and C.M. and CNPq grant to M.B.C.S. (Proc. No.306018/2013-6).
7. REFERENCES
Agetsuma, N. (2001). Relation between age-sex classes and dietary selection of wild Japanese monkeys. Ecological Research. 16, 759-763. [ Links ]
Altmann, J. (1974). Observational study of behavior: Sampling methods. Behaviour. 49, 227-265. [ Links ]
Altmann, S.A. (1998). Foraging for survival: yearling baboons in Africa. University of Chicago Press. [ Links ]
Bales, K.L., French, J.A., Hostetler, C.M. & Dietz, J.M. (2005). Social and reproductive factors affecting cortisol levels in wild female golden lion tamarins (Leontopithecus rosalia). American Journal of Primatology. 67, 25-35. [ Links ]
Bell, R.H.V. (1971). A grazing ecosystem in the Serengeti. Scientific American. 225, 86-93. [ Links ]
Behie, A.M., Pavelka, M.S.M. & Chapman, C.A. (2010). Sources of variation in fecal cortisol levels in howler monkeys in Belize. American Journal of Primatology. 72, 600-606. [ Links ]
Bercovitch, F.B. & Ziegler, T.E. (2002). Current topics in primate Socioendocrinology. Annual Review of Anthropology. 31, 45-67. [ Links ]
Cavigelli, S.A. (1999). Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta. Animal Behavior. 57, 935- 944. [ Links ]
Chapman, N. H., Bonnet, J., Grivet, L., Lynn, J., Graham, N., Smith, R., ... & King, G. J. (2012). High-resolution mapping of a fruit firmness-related quantitative trait locus in tomato reveals epistatic interactions associated with a complex combinatorial locus. Plant Physiology, 159(4), 1644-1657. [ Links ]
Chrousos, G.P. & Gold, P.W. (1992). The concepts of stress and stress system disorders. Journal of the American Medical Association. 267, 1244-1252. [ Links ]
Clymer, G.A. (2006). Foraging responses to nutritional pressures in two species of cercopithecines: Macaca mulatta and Papio ursinus. Thesis, Georgia State University. [ Links ]
Cunha, M.S., Vivacqua, C., Fernandes, L.C. & Sousa, M.B.C. (2007). Annual variation in plasma cortisol levels in common marmosets, Callithrix jacchus. Biological Rhythm Research. 38, 373-381. [ Links ]
Dias, A.C., Custodio Filho, A., Franco, G.A.D.C. & Couto, H.T.Z. (1995). Estrutura do componente arbóreos em um trecho de floresta pluvial atlântica secundária - Parque Estadual Carlos Botelho. Revista do Instituto Florestal. 7, 125-155. [ Links ]
Di Fiore, A. & Suarez, S. A. 2007. Route-based travel and shared routes in sympatric spider and woolly monkeys: cognitive and evolutionary implications. Animal Cognition, 10, 317e329. [ Links ]
Emery Thompson, M., Muller, M.N., Kahlenberg, S.M., & Wrangham, R.W. (2010). Dynamics of social and energetic stress in wild female chimpanzees. Hormones and Behavior, 58(3), 440-449. [ Links ]
Emery Thompson, M., & Georgiev, A.V. (2014). The high price of success: costs of mating effort in male primates. International Journal of Primatology, 35(34), 609-627. [ Links ]
Ganzhorn, J. U. (2002). Distribution of a folivorous lemur in relation to seasonally varying food resources: integrating quantitative and qualitative aspects of food characteristics. Oecologia,131(3), 427-435. [ Links ]
Garber, P. A. (1988). Diet, foraging patterns, and resource defense in a mixed species troop of Saguinus mystax and Saguinus fuscicollis in Amazonian Peru. Behaviour, 105(1), 18-34. [ Links ]
Girard, I., and Garland, T., Jr. 2002. Plasma corticosterone response to acute and chronic voluntary exercise in female house mice. Journal of Applied Physiology, 92, 1553-1561. [ Links ]
Izar, P. (2004). Female social relationship of Cebus apella nigritus in a South eastern Atlantic forest: an analysis through ecological models of primate social evolution. Behaviour. 141, 71-99. [ Links ]
Izar, P., Stone, A., Carnegie, S.D. & Nakai, E.S. (2009). Sexual selection, female choice and mating systems, in: Garber, P., Estrada, A., Bicca-Marques, J.C., Heymann E., and Strier, K. B. (Eds.), South American Primates: Testing New Theories in the Study of Primate Behavior, Ecology, and Conservation, New York: Springer Press, pp. 157-189. [ Links ]
Izar, P., Verderane, M. P., Peternelli-dos-Santos, L., Mendonça-Furtado, O., Presotto, A., Tokuda, M. & Fragaszy, D. (2012). Flexible and conservative features of social systems in tufted capuchin monkeys: comparing the socioecology of Sapajuslibidinosus and Sapajus nigritus. American Journal of Primatology. 73, 1-17. [ Links ]
Kleiber, M. (1961) The Fire of Life: an introduction to animal energetics. New York: Wiley. [ Links ]
Kriegsfeld, L.J. & Silver, R. (2006). The regulation of neuroendocrine function: timing is everything. Hormones and Behavior. 49, 557-574. [ Links ]
Lahoz, M.M., Nagle, C.A. & Porta, M. (2007). Cortisol response and ovarian hormones in juvenile and cycling female Cebus monkeys: effect of stress and dexamethasone. American Journal of Primatology. 69, 551-561. [ Links ]
Lindsay, J.R. & Nieman, L.K. (2005). The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocrine Reviews. 26, 775-799. [ Links ]
Lynch, J.W., Ziegler, T.E. & Strier, K.B. (2002). Individual and seasonal variation in fecal testosterone and cortisol levels of wild tufted capuchin monkeys, Cebus apella nigritus. Hormones and Behavior. 41, 275-287. [ Links ]
Lynch, J. W., & Rímoli, J. (2000). Demography of a group of tufted capuchin monkeys (Cebus apella nigritus) at the Estação Biológica de Caratinga, Minas Gerais, Brazil. Neotropical Primates, 8(1), 44-49. [ Links ]
McEwen, B. S. (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33-44. [ Links ]
McEwen, B.S. & Wingfield, J.C. (2003). The concept of allostasis in biology and biomedicine. Hormones and Behavior. 43, 2-15. [ Links ]
McEwen, B.S. & Wingfield, J.C. (2007). Allostasis and allostatic load, In: Fink, G. (Ed.), Encyclopedia of Stress, second edition. Academic Press, New York, pp. 135-141. [ Links ]
McEwen, B.S. & Wingfield, J.C. (2010). "What's in a name? Integrating homeostasis, allostasis and stress". Hormones and Behavior. 57, 105-111. [ Links ]
Mendonça-Furtado, O., Edaes, M., Palme, R., Rodrigues, A., Siqueira, J., & Izar, P. (2014). Does hierarchy stability influence testosterone and cortisol levels of bearded capuchin monkeys (Sapajus libidinosus) adult males? A comparison between two wild groups. Behavioural processes, 109, 79-88. [ Links ]
Moreira, C. M. (2010). Análise endócrino-comportamental dos macacos-prego (Cebus nigritus) que habitam o Parque Estadual Carlos Botelho. MSc. thesis. University of São Paulo, Brazil. [ Links ]
Muller, M.N., & Wrangham, R.W. (2004). Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behavioral Ecology and Sociobiology, 55, 332-340. [ Links ]
Nakai, E. S., (2007). Fissão-fusão em Cebus nigritus: flexibilidade social com estratégia de ocupação de ambientes limitantes. MSc. thesis. University of São Paulo, Brazil. [ Links ]
Oftedal, O. T. (1991). The nutritional consequences of foraging in primates: the relationship of nutrient intakes to nutrient requirements. Philosophical Transactions of the Royal Society Biological. 334, 161-170. [ Links ]
Peternelli dos Santos, L.C. (2010). Diferenças sexo/etárias do Forrageamento de Cebus nigritus em área de Mata Atlântica. MSc. thesis. University of São Paulo, Brazil. [ Links ]
Pinheiro, J., Bates, D., DebRoy, S., & Sarkar, D. (2014). R Core Team (2014) nlme: Linear and Nonlinear Mixed Effects Models. R package version3.1-115; 2014. [ Links ]
Presotto, A. & Izar, P. (2010). Spatial reference of black capuchin monkeys in Brazilian Atlantic Forest: egocentric or allocentric? Animal Behavior.80, 125-132. [ Links ]
Romero, L.M., Dickens, M.J. & Cyr, N.E. (2009). The reactive scope model - a new model integrating homeostasis, allostasis, and stress. Hormones and Behavior. 55, 375-389. [ Links ]
Sapolsky, R.M. (1983). Individual differences in cortisol secretory patterns in the wild baboon: role of negative-feedback sensitivity. Endocrinology. 113, 2263-2268. [ Links ]
Sapolsky, R.M. (1986). Endocrine and behavioral correlates of drought in wild olive baboons (Papio anubis). American Journal of Primatology. 11, 217-227. [ Links ]
Sapolsky, R.M., Romero L.M. & Munck, A.U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 21, 55-89. [ Links ]
Schoof, V.A.M., Jack, K.M., & Ziegler, T.E. (2014). Male Response to Female Ovulation in White-Faced Capuchins (Cebus capucinus): Variation in Fecal Testosterone, Dihydrotestosterone, and Glucocorticoids. International Journal of Primatology, 35(34), 643-660. [ Links ]
Setchell, J.M., Smith, T., Wickings, E.J. & Knapp, L.A. (2008). Factors affecting fecal glucocorticoid levels in semi-free-ranging female mandrills (Mandrillus sphinx). American Journal of Primatology. 70, 1-10. [ Links ]
Setchell, J.M., Smith, T.E., Wickings, E.J. & Knapp, L.A. (2010). Stress, social behaviour, and secondary sexual traits in a male primate. Hormones and Behavior58(5), 720-728. [ Links ]
Sousa, M.B.C. & Ziegler, T.E. (1998). Diurnal variation on the excreted patterns of fecal steroids in common marmosets (Callithrix jacchus) females. American Journal of Primatology. 46, 105- 117. [ Links ]
Strier, K. B., Ziegler, T. E. & Wittwer, D. J. (1999). Seasonal and social correlates of fecal testosterone and cortisol levels in wild male muriquis (Brachyteles arachnoides). Hormones and Behavior. 35, 125-134. [ Links ]
Taira, J., (2007). Consumo de palmito juçara (Euterpe edulis Mart.) por macacos-prego (Cebus nigritus): estratégia de forrageamento ótimo ou requinte de um gourmet? MSc. thesis. University of São Paulo, Brazil. [ Links ]
Tardif, S.D., Ziegler, T.E., Power, M. & Layne, D.G. (2005). Endocrine changes in full-term pregnancies and pregnancy loss due to energy restriction in the common marmoset (Callithrix jacchus). The Journal of clinical endocrinology and metabolism. 90, 335-339. [ Links ]
Torres-Farfan, C., Valenzuela, F.J., Ebensperger, R., Mendez, N., Campino, C., Richter, H.G., Valenzuela, G.J. & Seron-Ferre, M. (2008). Circadian cortisol secretion and circadian adrenal responses to ACTH are maintained in dexamethasone suppressed capuchin monkeys (Cebus apella). American Journal of Primatology.70, 93-100. [ Links ]
Weingrill, T., Gray, D.A., Barrett, L. & Henzi, S.P. (2004). Fecal cortisol levels in free-ranging female chacma baboons: relationship to dominance, reproductive state and environmental factors. Hormones and Behavior. 45, 259-269. [ Links ]
Wingfield, J.C. & Ramenofsky, M. (1999). Hormones and the behavioral ecology of stress. in: Baum, P.H.M. (Ed.), Stress Physiology in Animals. CRC Press, Sheffield, pp. 1-51. [ Links ]
Ziegler, T.E., Scheffler, G. & Snowdon, C.T. (1995). The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Hormones and Behavior. 29, 407-424. [ Links ]
Ziegler, T.E., Scheffler, G., Wittwer, D.J., Schultz-Darken, N., Snowdon, C.T. & Abbott, D.H. (1996). Metabolism of reproductive steroids during the ovarian cycle in two species of callitrichids, Saguinus oedipus and Callithrix jacchus, and estimation of the ovulatory period from fecal steroids. Biology of Reproduction. 54, 91-99. [ Links ]