Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
International Journal of Psychological Research
Print version ISSN 2011-2084
int.j.psychol.res. vol.9 no.2 Medellín Jul./Dec. 2016
https://doi.org/10.21500/20112084.2326
DOI: http://dx.doi.org/10.21500/20112084.2326
Research
Dissociation of Procedural and Working Memory in Pigeons (Columba livia)
Disociación de la memoria de procedimental y de trabajo en Palomas (Columba livia)
Walter T. Herbransona*
a Whitman College, Walla Walla, United States of America.
* Corresponding author: Walter Herbranson, Department of Psychology, Whitman College, 345 Boyer Ave, Walla Walla, WA 99362 Email Address: herbrawt@whitman.edu
Article history:
Received: 20-04-2016. Revised: 20-06-2016. Accepted: 23-06-2016
ABSTRACT
A new method was developed to concurrently investigate procedural memory and working memory in pigeons. Pigeons performed a sequence of keypecks across 3 response keys in a serial response task, with periodic choice probes for the location of a recently produced response. Procedural memory was operationally defined as decreasing response times to predictable cues in the sequence. Working memory was reflected by accurate responses to the choice probes. Changing the sequence of required keypecks to a random sequence interfered with procedural memory in the form of slowed response times, but did not prevent pigeons from effectively using working memory to remember specific cue locations. Conversely, changing exposure duration of to a cue location influenced working memory but had no effect on procedural memory. Double dissociations such as this have supported the multiple systems approach to the study of memory in cognitive psychology and neuroscience, and they encourage a similar approach in comparative psychology.
Key words: pigeon, response time, serial learning, procedural memory, working memory.
RESUMEN
Un nuevo método ha sido desarrollado para investigar la memoria procedimental y la memoria de trabajo en palomas. Las palomas realizan una secuencia de claves de picoteo a través de 3 respuestas clave en una tarea de respuestas seriales, probando periódicamente la elección por ubicación de respuestas producidas recientemente. La memoria procedimental fue operacionalmente definida como la disminución de los tiempos de reacción para las señales predecibles en la secuencia. La memoria de trabajo fue reflejada en las respuestas correctas en las pruebas de selección. Cambiar la secuencia de claves de picoteo requerida a una secuencia aleatoria interfirió con la memoria procedimental lentificando los tiempos de reacción, pero no impidió que las palomas usaran eficazmente la memoria de trabajo para recordar lugares específicos. Por el contrario, cambiar la duración de exposición de un lugar particular, influyó en la memoria de trabajo pero no tuvo efecto en la memoria procedimental. Esta doble disociación, ha contribuido al enfoque de sistemas múltiples para el estudio de la memoria en la psicología cognitiva y las neurociencias, y fomentan un enfoque similar en la psicología comparada.
Palabras clave: paloma, tiempo de reacción, aprendizaje seriado, memoria procedimental, memoria de trabajo.
1. INTRODUCTION
The field of comparative cognition has thrived by blending theories and concepts derived from cognitive psychology with methods and techniques independently developed to study animal behavior. An example of this synergy comes from the study of memory: a powerful apparatus for studying spatial learning and memory in animals is the radial arm maze (Olton & Samuelson, 1976), and its results are readily interpreted in terms of classic models of memory from cognitive psychology (e.g., Atkinson & Shiffrin, 1968). The radial arm maze consists of a number of arms (usually eight) extending from a central platform, and an animal is allowed to explore each. When the same subset of arms is baited with food items across sessions, rats use various forms of memory to visit each of the baited arms before the unbaited arms.
The maze yields the kind of objective, behavior-based measures of performance necessary for the study of animal learning, but also connects with well-established principles of cognition and memory. For example, revisiting a previously explored arm during the same session is considered to be an error of working memory (or short-term memory): the short duration, limited capacity buffer that is used to temporarily store and manipulate information (Baddeley & Hitch, 1974). In contrast, visiting an unbaited arm before any of the baited arms is considered to be an error of reference memory (or long-term memory): the durable, high-capacity repository of events and semantic information. The radial arm maze is remarkably flexible and has been used successfully to test memory in a variety of animals, including rats (Olton & Samuelson, 1976), gerbils (Wilkie & Slobin, 1983), hamsters (Jones et al., 1990), pigeons (Roberts & Van Veldhuizen, 1985), dogs (Macpherson & Roberts, 2010), pigs (Laughlin & Mendl, 2000), tortoises (Wilkinson, Chan & Hall, 2007), Siamese fighting fish (Roitblat, Tham & Golub, 1982), zebrafish (Colwill, Raymond, Ferreira & Escudero, 2005), and human children (Mennenga et al., 2014). Such flexibility is an appealing feature for any task in the arsenal of comparative psychologists, as inter-species comparisons can be planned and hold the potential to support meaningful inferences about the ultimate origins of the memory systems in question.
The connections between performance on the radial arm maze and models of memory is further supported by physiological, neurochemical, and task manipulations, using the powerful logic of double dissociation. In a double dissociation, one variable interferes with a specific cognitive process while leaving a different cognitive process unaffected. A second variable has the complimentary pattern of effects on the two cognitive processes. For example, Packard, Hirsh and White (1989) showed that on the 8-arm radial maze, fimbria-fornix lesions interfered with reference memory, but not working memory in rats, while caudate lesions interfered with working but not reference memory. Similarly, Kay, Harper and Hunt (2010) produced a neurochemical double dissociation on the radial arm maze, with MDMA and scopolamine interfering with working and reference memory, respectively. Non-physiological task manipulations can also affect performance in the same ways. The serial position curve (Murdock, 1962), a classic finding in cognitive psychology assumed to be a product of both working and reference memory can be replicated using the radial arm maze (Bolhuis & VanKampen, 1988). Furthermore, the component primacy and recency effects can be separately manipulated by factors that suggest similar memory processes. These kinds of double dissociations not only help to link cognitive processes with specific brain functions, but more fundamentally show the independence of those cognitive processes.
While working and reference memory constitute a fundamental distinction, modern theories of memory go far beyond these two, and dissociations are not limited to just these two varieties of memory (See Tulving, 1985). Decoteau and Kesner (2000) for example, used the aforementioned radial arm maze to dissociate procedural and declarative memory. Procedural memory is an implicit form of memory, involving how to perform a complex action or skill, whereas declarative memory refers to memories that can be explicitly accessed and declared. In their task, rats learned to visit a sequence of arms using a training procedure designed to recruit either procedural or declarative memory. In the procedural version, doors to baited arms were automatically opened in a specific sequence, and rats collected rewards more quickly as they learned to efficiently perform the resulting sequence (or procedure) of arm traverses. In the declarative version, rats learned to orient toward (or declare) the next arm in a learned sequence before it was opened. Rats learned both versions of the task, and more importantly, showed a double dissociation between hippocampal lesions (affecting declarative but not procedural memory) and medial caudoputamen lesions (affecting procedural but not declarative memory).
Decoteau & Kesner's measure of implicit memory was inspired by the serial response time (SRT) task, originally developed by Nissen and Bullemer (1987) to investigate procedural memory in humans. In an SRT experiment, different responses are cued by individual targets appearing in corresponding locations on a display. Participants respond to each target as it appears by pressing a corresponding button. When targets appear in a fixed, perpetually repeating sequence, response times become faster as training progresses. Subsequently, if targets appear in the same set of possible locations, but sequenced randomly, response times immediately slow, indicating that response time facilitation was due to learning about the consistencies in the repeating pattern, and not to familiarity with the apparatus or other general features of the method. When questioned about the sequence, participants are usually (though not always) unaware that there was a repeating pattern, and response time facilitation is seen whether or not a participant did notice any pattern. Hunt and Aslin (2001) further explored the basis for implicit SRT learning, and found that response time facilitation was due to participants' use of local statistical information. That is, human participants responded more quickly to some locations than others, with faster response times to those locations than could be more accurately predicted based on the most recently cued responses.
Froehlich, Herbranson, Loper, Wood, and Shimp (2004) developed a parallel SRT procedure for pigeons, and produced the same general pattern of results. Birds responded to cues (illuminated response keys) in an operant chamber by pecking the lit keys themselves. When target locations were determined by a specific repeating sequence, response times were faster than when they were randomly generated. Furthermore, pigeons appeared to respond quickly based on acquisition of the same kind of local statistical information that underlies SRT learning in humans.
While SRT learning is usually considered a form of procedural learning, procedural memory need not be the only memory system involved when learning to perform sequences of responses. Consider that an individual learning a structured sequence could be partly or completely aware of the structure of the sequence as it is being performed. Curran and Keele (1993) reported that some of their human participants were in fact able to articulate at least part of the sequence at the end of training, indicating that they possessed some accessible representation of the sequence (i.e., available to their working memory), in addition to the procedural memory established through performance. In fact, several lines of research point to parallel memory processes in SRT tasks (Willingham & Goedert-Eschman, 1999).
While Froehlich et al. (2004) made no effort to measure working memory in their SRT task, pigeons would also seem to be capable of using working memory to remember recently emitted responses. Shimp & Moffitt (1974), for example, presented pigeons with a list of stimulus-response pairs (the study phase), and following a delay, probed them (the test phase) for the location of one of the responses made during the study phase. Pigeons responded accurately, and results were consistent with the characteristics of working memory: the retention interval and serial position of the probed response produced decay and recency effects characteristic of working memory.
If pigeons do utilize both working and procedural memory in an SRT task, then factors that are known to affect those memory systems ought to affect performance in different and predictable ways. The present experiment is a variation of the SRT task, intended to explore both procedural and working memory, using the same kind of double-dissociation logic that has been used to investigate memory systems in rats using the radial arm maze. The task is similar to established pigeon versions of the SRT procedure (Froehloch et al., 2004; Herbranson & Stanton, 2011, Herbranson, Xi & Trinh, 2014), but with a periodic memory probe requiring working memory for prior responses. The memory probes should require working memory because pigeons must keep the probed location in the working memory buffer even while responding to intervening cues. Response time facilitation to the structured sequence on the other hand, should reflect procedural memory, as pigeons are expected to learn to respond more quickly by performing the required responses in an efficient manner. If these hypotheses are correct, response time facilitation and probe accuracy should be dissociable by manipulating task factors that specifically tax either working or reference memory. In particular, sequence structure is known to affect procedural memory: changing a structured response sequence to a random one is the standard manipulation that demonstrates procedural memory in an SRT task (but would not be expected to affect pigeons' working memory). Similarly, exposure duration is known to enhance working memory for a cue (but would not be expected to affect procedural memory). Thus, there is an expectation of a double dissociation between working memory (accurate responses to the probes) and procedural memory (response time facilitation) components of the task when these factors are separately manipulated.
2. METHOD
2.1 Animals
Five white carneaux pigeons (Columba livia) were obtained from the Palmetto Pigeon Plant (Sumter, SC). Each was maintained at approximately 80% of free-feeding weight (Poling, Nickel & Alling, 1990), with supplemental grain provided as needed in home cages after daily experimental sessions. Birds were housed individually in standard pigeon cages with free access to water and grit, in a colony room with a 14:10 hour light-dark cycle. All experimental sessions took place during the light cycle at approximately the same time 5-6 days per week.
2.2 Apparatus
Five BRS/LVE (Laurel, MD) operant chambers (Cubicle SEC-002 with response panel PIP-016) were used. Chambers were interfaced to a personal computer, which controlled all experimental events and recorded data.
2.3 Pretraining
Birds were pretrained in sessions consisting successively of habituation, magazine training, and autoshaping (Brown & Jenkins, 1968). Stimuli during autoshaping consisted of white or red lights illuminating either the center, left, or right response key.
Following shaping, the task as described below was begun, with 10 trials per session featuring a 1-second observation period, and a randomly generated two-response sequence. The number of daily trials, observation period, and sequence length were then gradually increased until reaching the final values described below.
2.4 Procedure
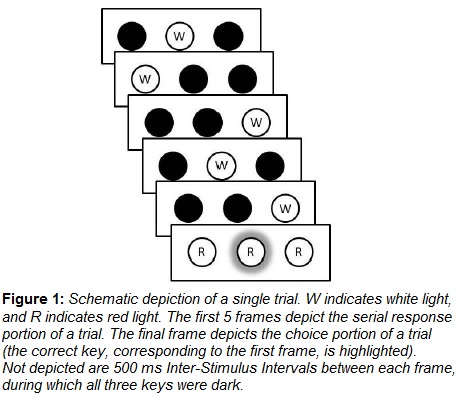
Birds completed 75 trials in each daily session. Each trial consisted of two phases, as illustrated in Figure 1. In the first phase (top 5 panels of Figure 1), birds completed a series of five key pecks, each of which was cued by the illumination of one of the three keys in the operant chamber with white light. Once an illuminated key was pecked, that key was immediately darkened and the next key in the sequence was illuminated following a 500 ms delay, until all five responses in the sequence had been completed. In the second phase of each trial (bottom panel of Figure 1), all three keys were illuminated with red light, and reinforcement in the form of approximately 3 s access to mixed grain was provided, contingent on pecking the first key the preceding sequence of 5 responses.
2.5 Conditions
Three conditions were run, for 212 days, 10 days, and 120 days respectively. The first condition included pretraining, and continued until pigeons performed at a consistently high level of accuracy, as determined by the experimenter. The 120 days of the third condition consisted of 12 blocks of 10 days each, with varying parameters as described below.
2.5.1 Condition 1 (Structured Sequences).
The response sequence used for the structured condition was C-L-R-C-R-L-. Note that the sequence had no designated first or last item, but was cyclical, with the last listed location (L) followed by the first (C). Each trial began at one of the six positions in the sequence (randomly determined by the computer on each trial), yielding six possible patterns of five responses on each trial, depending on the starting position (C-L-R-C-R, L-R-C-R-L, R-C-R-L-C, C-R-L-C-L, R-L-C-L-R, and L-C-L-R-C). Sequences of five responses were used on each trial (rather than six) so that the choice response would not match a continuation of the repeating pattern. In order to facilitate working memory for the first response, there was a required 5-second exposure duration for the first keylight presented on a trial. Subsequent cues had no minimum exposure durations.
2.5.2 Condition 2 (Random Sequences).
During Condition 2, response sequences were no longer structured, but consisted of five randomly generated target locations (with p = .33 for each of the left, center, and right keys). The remainder of a trial was the same as in Condition 1, with reinforcement again solely contingent on pecking the first illuminated key in the preceding five-response sequence.
2.5.3 Condition 3 (Manipulation of Exposure Duration).
Following completion of Condition 2, sequences were again generated from the same structured sequence used during Condition 1. Every 10 days, the required exposure duration to the first location on a trial was reduced from its initial value of 5 seconds, using the following values: 5.00, 4.00, 3.00, 2.50, 2.00, 1.50, 1.00, 0.75, 0.50, 0.25, 0.00). Finally, there was a 10-day replication of the initial 5-second exposure duration.
3. RESULTS
3.1 Condition 1
3.1.1 Choice Accuracy (Working Memory).
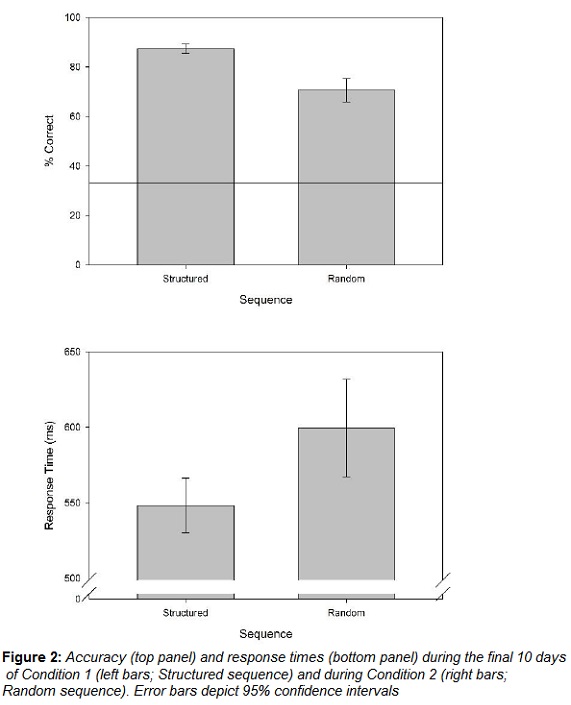
Median response times and mean choice accuracy during the final 10 days of Condition 1 can be seen in Figure 2 (left bars in both panels). Mean accuracy to choose the correct key at the end of a trial was 87.41%, CI = [85.52, 89.31]. The confidence interval confirms that this level of accuracy is greater than chance accuracy of 33% (d = 31.80).
3.1.2 Response Times (Procedural Memory).
The mean median response time over the final 10 days of Condition 1 was 548.15 ms, CI = [530.10, 566.20].
3.2 Condition 2
3.2.1 Choice Accuracy (Working Memory).
Median response times and mean choice accuracy during Condition 2 can be seen in Figure 2 (right bars in both panels). Mean accuracy to choose the correct key at the end of a trial was 70.61%, CI = [65.86, 75.36]. The confidence interval again falls entirely above chance accuracy of 33% (d = 9.75).
3.2.2 Response Times (Procedural Memory).
The mean median response time over the entire 10 days of Condition 2 was 599.47 ms, CI = [567.22, 631.72].
3.2.3 Global Response Time Facilitation Effect.
Procedural learning in serial response time tasks is usually indicated by a comparison of response times between structured and random conditions. Median response times were 51.32 ms faster over the final 10 days of the structured Condition 1 than over the 10 days constituting the random Condition 2, CI = [20.57, 82.07] d = 2.07. This statistically reliable difference corresponds to the global facilitation effect that indicates procedural learning in a serial response time task.
3.3 Condition 3
3.3.1 Choice Accuracy (Working memory)
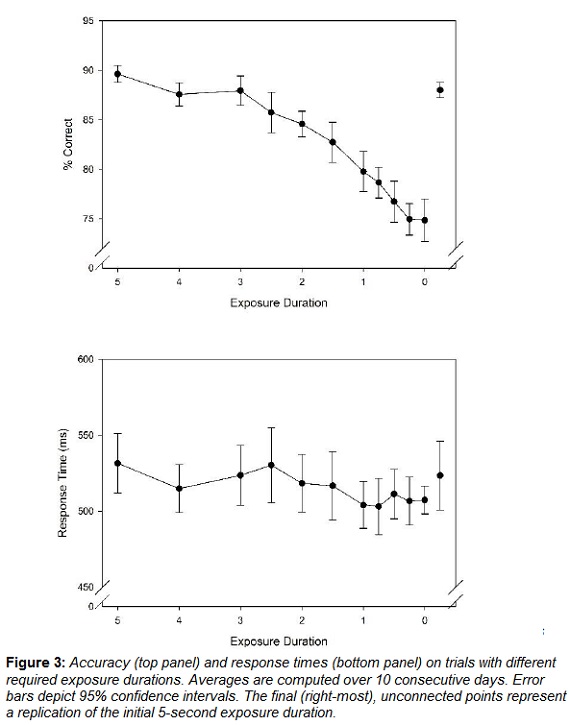
Median response times and mean choice accuracy for each of the 12 exposure durations in Condition 3 can be seen in Figure 3. A repeated-measures ANOVA indicates that there were significant differences in accuracy across the different exposure durations, F(11, 99) = 52.83, p < .01. In particular, accuracy dropped as exposure durations became shorter. Note that accuracy levels for all exposure durations were reliably better than chance accuracy of 33%, as indicated by the 95% Confidence Intervals displayed in the top panel of Figure 3. Also, while brevity of exposure is confounded with the sequence of conditions, note that the final condition, a replication of the initial 5-second exposure duration matches closely the initial condition, also featuring a 5-second exposure duration.
3.3.2 Response Times. (Procedural Memory)
There were no significant response time differences across the different exposure durations, F(11, 99) = 1.45, p = .16.
3.3.3 Estimation of Working and Reference Memory Contributions to Correct Choices.
A simple model of choice accuracy can be used to compute the probability of responding correctly based on two parameters (see Equation 1). The probability of correctly responding (c) is equal to the probability that a bird has the correct cued location active in working memory (w), plus the probability that a bird does not have the cued location in working memory (1-w) but responds correctly anyway by guessing (g).
Note that while g is termed “guessing”, it does not imply that birds necessarily respond randomly, only that they are responding without the benefit of an accurate cue active within working memory. That is, the value of g could be .33 (corresponding to a random choice from among the three available response keys), but it need not be. Consider that during Conditions 1 and 3 (when sequences were structured), a bird might acquire and make use of associations between the final response (or responses) in a sequence, and the correct response to the upcoming probe. For example, if the final response during the first portion of a trial is L, the correct cue can only be L (if the sequence was L-R-C-R-L) or C (if the sequence was C-R-L-C-L). Thus, with knowledge of the 6 possible sequences, one could guess with g = .50 (by eliminating the necessarily incorrect R option). Furthermore, memory of the final two responses could yield perfect accuracy (g = 1.00). For example, only one of the six sequences ends with the two-key sequence R-L, and it always begins with the same cue (L). Thus, birds could (potentially) perform quite accurately, even without the use of working memory. Nevertheless, such a strategy would become quite apparent in the randomly sequenced Condition 2 because it would necessarily lead to chance performance.
In Condition 2, the probability of responding correctly without having the correct cued location active in working memory (g) can only be .33, no matter what additional associations a bird has acquired (because cues during the SRT portion of a trial were randomly generated). If so, then given the overall accuracy of .706 during Condition 2, solving Equation 1 for w implies the probability that a bird would have the cue in working memory during the choice portion of a trial is .559. The additional correct responses (accounting for .706 - .559 = .147) would be cases in which the bird did not remember, but guessed correctly, with probability g = .33.
With this estimate of birds' working memory derived from the results of Condition 2, we can return to Condition 1. Given that all task details relevant to working memory were the same (such as exposure duration and the number of intervening cued responses), the demands on working memory should be the same. Thus, using the working memory probability just computed (w = .559), the probability of correctly guessing must be g = .714 in order to yield the overall accuracy (c = .874) obtained in Condition 1. Thus, we can conclude two things about birds' performance. First, they were apparently “guessing” with better than chance accuracy (i.e., g > .33), and this presumably reflected the kinds of learned associations described earlier. Second, because the guessing parameter still fell outside of the confidence interval for overall accuracy in Condition 1, accuracy cannot be accounted for entirely by these learned associations. The additional accuracy is attributable to working memory.
Further supporting the accuracy of this simple model is the fact that this guessing parameter (.714) was numerically very close to overall accuracy for the shortest exposure durations tested in Condition 3 (M = .750 for 0.25 s exposure duration and M = .749, for 0.00 s exposure duration). At these exposure durations, pigeons had little working memory for the sample (despite their relatively good overall accuracy), and were presumably relying almost exclusively on learned associations between the sequences and correct choices. It is important, however, that the working memory-independent guessing parameter still fell outside of the confidence intervals for even those shortest exposure durations (CI = [.734, .766] and CI = [.727, .770], meaning that there was still a reliable influence of working memory. Working memory accuracy at longer exposure durations was even better, implying that birds relied more heavily on working memory in those conditions, and demonstrating that working memory varied as a function of exposure duration.
4. DISCUSSION
Birds learned to perform structured sequences of responses quickly, and also to remember the initial cued locations that began each trial. Furthermore, the two manipulated variables in Conditions 2 and 3 separately affected each of these two dependent measures of memory. Specifically, when the structured sequence of cued responses was changed to a random sequence in Condition 2, response times (a measure of procedural memory) slowed significantly, but choice accuracy (a measure of working memory) remained significantly better than chance. Conversely, shortening exposure duration in Condition 3 significantly impaired accuracy, but had no effect on response times.
The difference between median response times in Conditions 1 and 2 parallels the operational definition of procedural learning characteristic of SRT tasks. This difference between the median response times during structured and random conditions, is termed global facilitation. This global effect does not necessarily reflect, but is often due to the different conditional predictabilities of elements within the sequence, termed local predictability. Herbranson & Stanton (2011), for example, used the sequence L-C-R-L-R-C-R-L-C-. Each of the three responses (left, center, and right) are equiprobable, appearing exactly thrice within each cycle of nine consecutive responses. However, the conditional probability of each location varies depending on the preceding cue. For example, the three instances of center responses (C) are twice followed by right responses (R) and once followed by a left response (L). Thus, the first order conditional probabilities for responses following center cues are p(R|C) = .67 and p(L|C) = .33. Using the same logic, one can compute second- and third-order local predictabilities that are dependent on the two or three preceding cues. Previous research indicates that both pigeons (Froehlich et al., 2004; Herbranson & Stanton, 2011) and humans (Hunt & Aslin, 2001) learn to perform sequences quickly by learning these local predictabilities, because some responses in the sequence (those with higher local predictabilities) contribute disproportionately to the faster median response time during structured conditions.
Response time facilitation in the present experiment was almost certainly based on second-order (or higher) local predictability. This is because the first-order local predictabilities for the sequence used (C-L-R-C-R-L-) were all identical and ambiguous, with values for the two possible keys that could follow any response being equal. For example, given a response on the left key, a subsequent response on the center key had the same conditional probability as a subsequent response on the right key (e.g., p(C|L) = p(R|L) = .50). All second order (and higher) predictabilities were completely certain (e.g., p(R|CL) = 1.00), as only one instance of each response pair appeared in the sequence. These features of the sequence used carry the implication that different elements within the sequence are not inherently more or less useful. Thus, the fine-grained analysis of local predictability often useful in SRT experiments is not informative in the present case for anything other than a study of between-subject variability.
The results reported here provide further support for the characterization of SRT learning as implicit, because learning (indicated by faster response times) was unrelated to the task that pigeons performed for food reward. That is, pigeons gained reinforcement for accurately remembering a previous key location, and not for performing the structured sequence quickly. Attention was presumably occupied with working memory for the initial item, and not with the memorization or rehearsal of the response sequence (which would have yielded no advantage).
The second measure of performance was recall of the initial response location, which required working memory. This result is consistent with results from experiments designed to explore working memory, such as delayed matching to sample, in which memory is usually worse after shorter exposure durations (White, 1985). In the reported experiment, shorter exposure durations also yielded worse accuracy, though accuracy always remained better than chance. Also supporting the interpretation of recall as reflective of working memory is the finding that it remained better than chance when the required response sequence was random, during Condition 2. This is critical because there were six specific sequences (each beginning in a different location), and each was associated with a specific correct response (dependent on the first cue in the sequence). Consequently, it would have been possible for birds to have performed accurately during Condition 1 not by remembering the initial location in working memory, but instead by utilizing learned associations between the specific six sequences and the appropriate response for each. Such a strategy however, could not work during Condition 2 when sequences were randomly generated. In Condition 2, only the use of working memory could support accurate performance, and birds' performance remained better than chance. It is worth noting however, that while accuracy was well above chance, it did drop during Condition 2. This is consistent with the simple model proposed for choice accuracy, in which accuracy is a function of both working and reference memory in the structured (but not random) conditions. Thus, it is likely that birds used specific learned associations to augment working memory during Condition 1 (as well as Condition 3). Roberts, Strang and Macpherson (2015) studied interactions between working and reference memory in pigeons using symbolic delayed matching to sample. They found that the two were independent and had a reciprocal relationship: Strengthening one of the two lead to a decrease in the other. The results from Experiment 3 are consistent with such an interpretation: as sample duration was reduced, working memory was weakened, but the influence of reference memory increased, keeping overall accuracy well above chance. However, even at the shortest sample durations, there was evidence of working memory, and the influence of working memory increased with sample duration.
The overall pattern of results reported here constitute a double dissociation: Sequence structure affected procedural memory but did not eliminate working memory, whereas exposure duration influenced working memory but had no effect on procedural memory. The primary implication is that working and procedural memory are separable cognitive processes in pigeons (though working memory may interact with reference memory under some circumstances). Furthermore, the task-based double dissociation displayed here suggests that future research could explore corresponding biologically-based double dissociations, thus making new connections with behavioral neuroscience. Diekamp, Kalt & Gunturkun (2002) localize pigeon working memory to the neostriatum caudolaterale. Thus, a lesion to that area might produce impaired cue recall, but normal response-time facilitation (as was seen in Condition 3 when exposure duration was manipulated). Procedural learning, in contrast, may rely on non-cortical structures such as the basal ganglia. Recall that Decoteau & Kenser (2000) found that lesions to rats' medial caudoputamen impaired a measure of procedural memory similarly inspired by SRT learning. Thus, striatal lesions might be expected to impair response time facilitation, but not working memory (as was seen in Condition 2, when sequence structure was manipulated). The new behavioral method presented here should facilitate testing these and other related hypotheses about the biological bases of memory systems in pigeons and potentially other animals.
Finally, these results show how comparative psychology had benefitted from, and can continue to benefit from, mutual connections with other areas of psychology. In this case, concepts derived from the study of human cognition (such as working and procedural memory) have led to experiments that otherwise would likely never occur (see Zentall, 2013). These experiments made use of methods developed separately to study animal behavior, and are here deployed in a novel context. Finally, the results can in turn be placed within a rich biological context, informed by both behavioral neuroscience and evolution. Like the radial arm maze, the procedure developed here makes use of a commonly used apparatus (the operant chamber) and methods (SRT and matching to sample), such that it should be a plausible method for investigating memory in a variety of species. SRT tasks have been successfully developed for pigeons (Froehlich et al., 2004), rats (Christie & Dalrymple-Alford, 2004), rhesus monkeys (Procyk, Dominey, Amiez, & Joseph, 2000), and cotton-top tamarins (Locurto, Gagne, & Levesque, 2009), If so, such cross species investigations hold considerable potential to elucidate the evolution of the procedural and working memory systems involved. Future research should continue to integrate these different contributors (cognitive processes, behavior, and biological psychology) to comparative psychology.
5. ACKNOWLEDGMENT
The author would like to thank Charlie Shimp for helpful comments on a previous draft of this paper. This research was supported by a grant from the National Institutes of Health.
6. REFERENCES
Atkinson, R. C., & Shiffrin, R. M. (1968). Chapter: Human memory: A proposed system and its control processes. In Spence, K. W., & Spence, J. T. The psychology of learning and motivation (Volume 2). New York: Academic Press. pp. 89-195. [ Links ]
Baddeley, A .D., & Hitch, G. (1974). Working memory. In G.H. Bower (Ed.), The psychology of learning and motivation: Advances in research and theory (Vol. 8, pp. 47-89). New York: Academic Press. [ Links ]
Bolhuis, J. J., & Van Kampen, H. S. (1988). Serial position curves in spatial memory of rats: Primacy and recency effects. The Quarterly Journal of Experimental Psychology, 40(2), 135-149. [ Links ]
Brown, P. L., & Jenkins, H. J. (1968). Autoshaping of the pigeon's keypeck. Journal of the Experimental Analysis of Behavior, 11, 1-8. [ Links ]
Christie, M.A., & Dalrymple-Alford, J. C. (2004).A new rat model of the human serial reaction time task: Contrasting effects of caudate and hippocampal lesions. Journal of Neuroscience, 24, 1034-1039. [ Links ]
Colwill, R., Raymond, M., Ferreira, L., & Escudero, H. (2005). Visual discrimination learning in zebrafish. Behavioral Processes, 70, 19-31. doi:10.1016/j.beproc.2005.03.001. [ Links ]
Curran, T. & Keele, S.W. (1993). Attentional and nonattentional forms of sequence learning. Journal of Experimental Psychology: Learning, Memory & Cognition, 19, 189-202. [ Links ]
DeCoteau, W. E., & Kesner, R. P. (2000). A double dissociation between the rat hippocampus and medial caudoputamen in processing two forms of knowledge. Behavioral neuroscience, 114(6), 1096. [ Links ]
Diekamp, B., Kalt, T. & Gunturkun, O. (2002). Working memory neurons in pigeons. Journal of Neuroscience, 22, 1-5. [ Links ]
Froehlich, A. L., Herbranson, W.T., Loper, J.D., Wood, D.M. & Shimp, C.P. (2004). Anticipating by pigeons depends on local statistical information in a serial response time task. Journal of Experimental Psychology: General, 133(1), 31-45. [ Links ]
Herbranson, W.T., Xi, P.M. & Trinh, Y.T. (2014). Spatial variability in serial response learning and performance by pigeons (Columba livia). International Journal of Comparative Psychology, 27(2), 280-294. [ Links ]
Herbranson, W.T. & Stanton, G.L. (2011). Flexible Serial Response Learning by Pigeons (Columba livia) and Humans (Homo sapiens). Journal of Comparative Psychology, 125(3), 328-340. [ Links ]
Hunt, R. H., & Aslin, R. N. (2001). Statistical learning in a serial reaction time task: Access to separable statistical cues by individual learners. Journal of Experimental Psychology: General, 130, 658-680. [ Links ]
Locurto, C., Gagne, M., & Levesque, K. (2009). Implicit chaining in cotton-top tamarins (Saguinus oedipus). Journal of Experimental Psychology: Animal Behavior Processes, 35, 116-122. [ Links ]
Jones, C., McGhee, R., & Wilkie, D. (1990). Hamsters (Mesocricetus auratus) use spatial memory in foraging for food to hoard. Behavioral Processes, 21, 179-187. doi:10.1016/0376-6357(90)90023-9. [ Links ]
Kay, C., Harper, D. N., & Hunt, M. (2010). Differential effects of MDMA and scopolamine on working versus reference memory in the radial arm maze task. Neurobiology of learning and memory, 93(2), 151-156. [ Links ]
Laughlin, K., & Mendl, M. (2000). Pigs shift too: Foraging strategies and spatial memory in the domestic pig. Animal Behavior, 60, 403-410. doi:10.1006/anbe.2000.1468. [ Links ]
Macpherson, K., & Roberts, W. A. (2010). Spatial memory in dogs (Canis familiaris) on a radial maze. Journal of comparative psychology, 124(1), 47. [ Links ]
Mennenga, S.E., Baxter, L.C., Grunfeld, I.S., Brewer, G.A., Aiken, L.S., Engler-Chiurazzi, E.B., Camp, B.W., Acosta, J.I., Braden, B.B., Schaefer, K.R. and Gerson, J.E. (2014). Navigating to new frontiers in behavioral neuroscience: traditional neuropsychological tests predict human performance on a rodent-inspired radial-arm maze. Frontiers in Behavioral Neuroscience, 8. [ Links ]
Murdock, B. (1962). Serial Position Effect of Free Recall. Journal of Experimental Psychology, 64(2), 482-488. doi:10.1037/h0045106. [ Links ]
Nissen, M.J. & Bullemer, P. (1987). Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology, 19, 1-32. [ Links ]
Olton, D.S. & Samuelson, R.J. (1976). Remembrances of places past: Spatial memory in rats. Journal of Experimental Psychology: Animal Behavior Processes. 2, 97-116. [ Links ]
Packard, M. G., Hirsh, R., & White, N. M. (1989). Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. The Journal of neuroscience, 9(5), 1465-1472. [ Links ]
Poling, A., Nickel, M. & Alling, K. (1990). Free birds aren't fat: Weight gain in captured wild pigeons maintained under laboratory conditions. Journal of the Experimental Analysis of Behavior, 53, 423-424. [ Links ]
Procyk, E., Dominey, P. F., Amiez, C., & Joseph, J.-P. (2000). The effects of sequence structure and reward schedule on serial reaction time learning in the monkey. Cognitive Brain Research, 9, 239-248. [ Links ]
Roberts, W.A., Strang, C., & Macpherson, K. (2015). Memory systems interaction in the pigeon: Working and reference memory. Journal of Experimental Psychology: Animal Learning and Cognition, 41(2), 152-162. [ Links ]
Roberts, W. A., & Van Veldhuizen, N. (1985). Spatial memory in pigeons on the radial maze. Journal of Experimental Psychology: Animal Behavior Processes, 11(2), 241. [ Links ]
Roitblat, H. L., Tham, W., & Golub, L. (1982). Performance of Betta splendens in a radial arm maze. Animal Learning & Behavior, 10(1), 108-114. [ Links ]
Shimp, C.P. & Moffitt, M. (1974). Short-term memory in the pigeon: Stimulus-response associations. Journal of the Experimental Analysis of Behavior, 22(3), 507-512. [ Links ]
Tulving, E. (1985). How many memory systems are there?. American psychologist, 40(4), 385. [ Links ]
White, K.G. (1985). Characteristics of forgetting functions in delayed matching to sample. Journal of the Experimental Analysis of Behavior, 44, 15-34. [ Links ]
Willingham, D.B., & Goedert-Eschmann, K. (1999). The relation between implicit and explicit learning: Evidence for parallel development. Psychological Science, 10, 531-534. [ Links ]
Wilkie, D. M., & Slobin, P. (1983). Gerbils in space: Performance in the 17-arm radial maze. Journal of the experimental Analysis of Behavior, 40(3), 301-312. [ Links ]
Wilkinson, A., Chan, H. M., & Hall, G. (2007). Spatial learning and memory in the tortoise (Geochelone carbonaria). Journal of Comparative Psychology, 121(4), 412. [ Links ]
Zentall, T. R. (2013), Comparative cognition: An approach whose time has come. Journal of the Experimental Analysis of Behavior, 100: 257-268. doi: 10.1002/jeab.35 [ Links ]