Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
International Journal of Psychological Research
Print version ISSN 2011-2084
int.j.psychol.res. vol.9 no.2 Medellín Jul./Dec. 2016
https://doi.org/10.21500/20112084.2339
Review
DOI: http://dx.doi.org/10.21500/20112084.2339
Sociosexual Interactions in Rats: Are They Relevant for Understanding Human Sexual Behavior?
Interacción Sociosexual en ratas: ¿son relevantes para comprender el comportamiento Sexual humano?
Xi Chua* and Anders Ågmoa
a Department of Psychology, University of Tromsø, Norway.
Article history:
*Corresponding author: Xi Chu, Department of Psychology, University of Tromsø, Huginbakken 32, 9037 Tromsø, Norway. Fax: +47 77 64 52 91. Tlf: +47 77 64 92 13. Email address: xi.chu@uit.no.
Received: 18-03-2016. Revised: 23-05-2016. Accepted: 26-05-2016.
ABSTRACT
When a prolonged observation of groups of rats in a seminatural environment is used as testing procedure, different behavioral patterns are shown compared with what observed in a pair housed in a small cage. Males and females copulate simultaneously, they show a promiscuously and random copulatory pattern. Females remain completely receptive from the first lordosis displayed in the period of behavioral estrus until the last. There is no reduction in paracopulatory behaviors and no increase in rejections towards the end of estrus. Female paracopulatory behavior and receptivity change in a most abrupt way at both initiation and termination of behavioral estrus. It appears that, in the seminatural environment, males copulate in bouts, and males do not pursue the females unless they are fully receptive. Non-sexual, social behavior including affiliative and nonaffiliative interaction among rats is rather unrelated to sexual activities in both sex.
Key words: representative design, seminatural environment, sexual behavior, social behavior, rat.
RESUMEN
Cuando una observación prolongada de grupos de ratas en ambientes seminaturales es usada como procedimiento de evaluación, diferentes patrones conductuales se muestran en comparación con lo que se observa con una pareja en una jaula pequeña. Machos y hembras copulan simultáneamente, muestran promiscuidad y un patrón aleatorio de copulación. Las hembras permanecen completamente receptivas desde su primera lordosis desplegada en el periodo de comportamiento de celo hasta el último. No hay una reducción de comportamientos para-copulatorios y no hay incremento en el rechazo hacia el final del celo. Las conductas para-copulatorias en hembras y la receptividad cambian de la manera más abrupta tanto al iniciarse como al terminarse la conducta de celo. Al parecer, en un ambiente seminatural, los machos copulan en peleas y no persiguen a las hembras a menos que ellas estén totalmente receptivas. Conductas sociales no sexuales, incluidas las interacciones afiliativas y no afiliativas entre ratas, no están relacionadas con actividades sexuales en ambos sexos.
Palabras clave: diseño representativo, ambiente seminatural, conducta sexual, conducta social y rata.
1. INTRODUCTION
In most laboratory studies of animal behavior, we do not want to limit our conclusions to the specific individuals observed. Rather we wish to generalize our findings to the population from which our experimental subjects were drawn. Likewise, we would often like to generalize our results to situations different from the specific procedure employed in the laboratory. These basic notions apply to all fields of behavioral inquiry, but we will focus on the field of rodent sexual behavior. Thus, if we happened to study the structure of copulatory behavior in ten opposite-sex pairs of rats, we would like to believe that the structure determined in our pairs would apply also to other pairs of rats. We assume that this is the case as long as we have a random sample of rats.
A different question is whether the structure of copulatory behavior in our pairs, observed for a short time in a standard observation cage, would be similar if we instead observed them for a long time in a large, complex environment. This we cannot know until we have performed such observations. We could also ask ourselves whether the structure of copulatory interactions observed in opposite-sex pairs would be similar to that observed when several males had the opportunity to copulate with the same female, or when several females copulated with the same male, or when several males and several females copulated with whomever they wished. Again, we would need to perform all these observations in order to determine if behavioral structures indeed are generalizable from one context to another.
Many years ago, it was suggested that in order to generalize research findings to all possible contexts we need to employ a representative design (Brunswik, 1955). In parallel to the need of a representative sample of individuals from the population we wish to generalize to, a representative design means a random sample of all the possible contexts we wish to generalize to. This is certainly quite impractical, particularly when applied to animal behavior, and when we wish to generalize to contexts outside the laboratory, for example to conditions in the wild. Nevertheless, the Brunswikian spirit has been applied to studies of animal behavior (Petrinovich, 1979 ; Petrinovich & Patterson, 1980). An approximation to a representative design in animal behavior studies can be achieved by including as many elements as possible of the animals’ natural habitat in the laboratory setting. This would at least make it possible to accurately describe complex behavior patterns and formulate hypotheses concerning the functional importance of the behavioral structures observed. Basing notions of function, or of adaptive value, on data from an entirely artificial context is extremely risky.
A representative design is particularly important when behavior is at the forefront. In studies of the neurobiological mechanisms controlling a behavior pattern, for example the lordosis posture in the female rodent or the display of paracopulatory behavior, the specific procedure is probably inconsequential. Silencing of the estrogen receptor α in the ventromedial nucleus of the hypothalamus will strongly reduce these behaviors both in a standard copulation test (Spiteri et al., 2010) and in a seminatural environment (Snoeren et al., 2015), just to mention an example.
The concept of representative design is equivalent to what frequently is called external validity. Sometimes the term ecological validity is used instead. This, however, is most unfortunate since ecological validity within the Brunswikian framework refers to the predictive reliability of a stimulus, and not to the generalizability of data from a specific experimental procedure (Brunswik & Kamiya, 1953). Regardless of this, we will begin with an overview of some of the non-representative designs used in studies of sociosexual behaviors in rats. Then we will outline some observations on the sociosexual behavior in a seminatural environment, incorporating the basic elements of rats’ natural habitat. We will also highlight several of the differences between our data and some notions derived from studies in non-representative procedures.
2. RATS’ NATURAL HABITAT: PHYSICAL AND SOCIAL ASPECTS
Rats are as cosmopolitan as humans. Their habitat range from the skyscrapers on Manhattan to the garbage dumps in Calcutta, and from the research stations in the Antarctic to the tropical rain forests of the Amazon. It is, consequently, impossible to make a specific description of rats’ natural, physical habitat. It could probably be maintained that any setup includes some of the physical elements of rats’ natural habitat, and that there is none that can include them all.
There seems to be far less variability in the social aspects of rats’ natural habitat. Wild rats live in groups, and occupy a rather extensive area for their daily activities and share elaborate burrows with tunnels and chambers (Calhoun, 1962a). A typical organization of a group of rats consists of several females, a small number of males, and offspring (McClintock, 1987). Studies involving wild rats are usually conducted in a field with a native rat pen. Their sociosexual interaction has been described earlier by Calhoun (1962a). Approximately one day prior to a wild female rat enters behavioral estrus, she drags her anogenital region over the soil around trees, bushes, and at both sides of a burrow to mark her scent. Shortly after, male rats notice the female scent and they mark their own scent as well. The female rat wanders beyond the limits of her normal home range and actively search males. No copulatory behavior is observed before she is fully receptive, but she copulates with multiple males during the entire period of estrus. As a consequence, many of the males involving in this group sex successfully copulate with the estrous female (Robitaille & Bouvet, 1976). In fact, multiple paternity appears to be common in wild rat populations (Miller, Russell, MacInnes, Abdelkrim, and Fewster, 2010).
3. EXAMPLES OF PROCEDURES COMMONLY USED IN THE LABORATORY
3.1 Standard copulation cage
The shape and size of the enclosure used for studying sexual behavior is varied, but it is generally rather small, e.g. a rectangular steel cage with a dimension of 40 × 60 cm was used by Ågmo (1997), and another cage of 50 × 80 cm was used by Madlafousek & Hliňák (1977). This setup is commonly used for studying social and sexual interactions between pairs of opposite sex individuals (Figure 1), and it is suitable to test for behaviors in both sexually naïve and experienced animals.
Compared with wild rats’ living conditions, observing the behavioral patterns of a pair of rats in this kind of cage cannot be considered to be an externally valid procedure. The number of available sexual partner in such behavioral tests in most of the cases is one. There are a couple of studies regarding sexual exhaustion, in which males were allowed to copulate with multiple females simultaneously in a standard copulation cage (e.g . Barto & Trojan, 1982; Tiefer, 1969). Prior to the first ejaculation more mounts and intromissions were observed in the presence of multiple receptive females than in that of a single female. The copulatory acts of the males were distributed among multiple females but not all females received ejaculations (Barto & Trojan, 1982 ; Tiefer, 1969). In addition, there are some differences in sociosexual interaction during copulation between in a pair context and in a situation of three males copulating with a single female in a standard cage (Chu & Ågmo, unpublished). The latencies to mount and intromission as well as the intercopulatory interval (mean of the interval between two consecutive copulatory acts displayed by a male) are longer in the multiple males’ situation than in the pair setting. Moreover, when there are 3 males copulating with one female compared to females in a pair setting, the females displayed less sniffing of males and more nose off. These are just two examples to illustrate the behavioral differences between observations of multiple males or females and a pair.
3.2 Incentive motivation testing arena
A motivation testing arena (Figure 2) can be a device with two small cages fitted to the outside wall of an oval arena. The small cages are diagonally opposed, and communicate with the arena through a double wire mesh. Outside the openings, a virtual area is defined as the incentive zone (Ågmo, 2003). This apparatus is usually used to test the subject’s sexual motivation or partner preference by placing different stimuli in the two small cages (e.g. Chu et al., 2008 ; Chu & Ågmo, 2008, 2016; Snoeren, Lehtimaki, & Agmo, 2012; Spiteri et al., 2010; Spiteri, Ogawa, Musatov, Pfaff, & Ågmo, 2012).
3.3 Three-compartments test box
Partner preference tests are also carried out in a test box with three compartments (60 x 30 x 40 cm, e.g. Slob, de Klerk, & Brand, 1987). Each mating chamber consists of a Plexiglas arena divided into three equal compartments using clear Plexiglas dividers. Each of the dividers has a small hole (usually about 5 cm in diameter) in each of the two bottom corners. A stimulus animal is placed in one lateral compartment and another stimulus animal in the other. Experimental animals can move freely from one compartment to the next through a small opening in the partitions separating the compartments while the stimulus animals are restrained in their compartments. Sometimes the experimental subject is allowed to physically interact with the stimulus animals, while they can also be separated by a wire mesh or something similar.
3.4 Pacing chamber
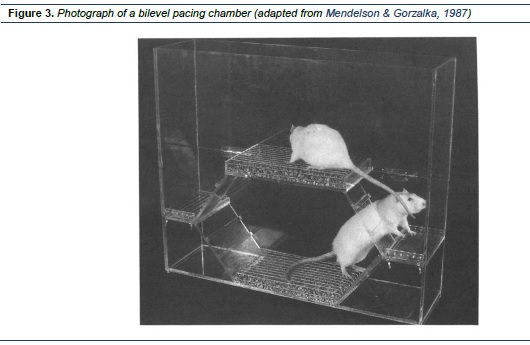
There are several kinds of pacing chambers. A bilevel observation chamber (Figure 3), made of Plexiglas, includes two levels with a platform centered above the floor. A set of ramps and a narrow landing at each end of the chamber allow the animals to move freely from one level to the other. A male rat is usually placed at the lower level, which allows the female to pace sexual interactions by entering male level (e.g. Mendelson & Gorzalka, 1987 ; Mendelson & Pfaus, 1989). A two-compartment chamber (also known as partitioned test cage or unilevel pacing chamber) is a small Plexiglas cage, which is equally divided by a removable barrier. A small hole (usually 5 cm in diameter) in the bottom center of the partition allows movement of the female from one side of the cage to the other and at same time the male is restrained to one side of the cage. Both bilevel and unilevel pacing chambers are used for examining female-controlled timing of sexual contacts with the male (e.g. Erskine, 1985; Pfaus, Smith, & Coopersmith, 1999).In summary, all of these commonly used procedures mentioned above require only a short duration of observation. Each of the research trials in many studies may only consist of one ejaculatory series.
4. EXAMPLES OF REPRESENTATIVE DESIGNS
4.1 Seminatural environment
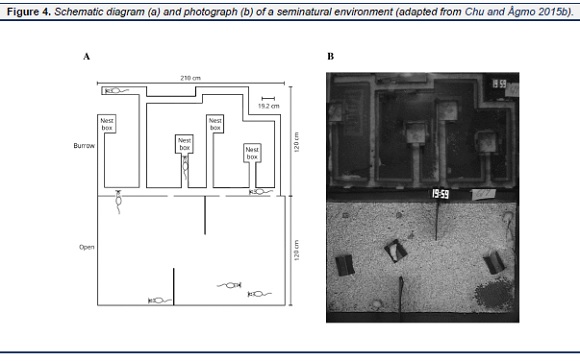
A seminatural environment may consist of a complex burrow system and an open area (Figure 4, for details see Chu & Ågmo, 2014). The one we have used in several studies measures 2.8 × 2.4 m, with a burrow consisting of several tunnels and 4 nest boxes. The design of the burrow is based upon descriptions of wild rat burrows (Calhoun, 1962a) with regard to the dimensions of tunnels and nest boxes. The burrows as well as the adjacent open area are similar to the apparatus used by McClintock and colleagues (McClintock & Adler, 1978 ; McClintock & Anisko, 1982; McClintock, Anisko, & Adler, 1982). There are 4 small openings between the burrow and the open area. Before the animals are introduced, the floor of the entire device is covered with aspen wood shavings. Twelve wood sticks and 3 plastic shelter huts are put in the open area, and nest building material is put in the nest boxes. About 3 kg of pellets are provided in a corner of the open area, and 4 water bottles are freely available in that corner. There is a 12:12 h light/dark cycle in the open area. During the dark phase, light intensity is about 1 lx at floor level. It is about 180 lx during the light phase. A light-blocking wall provides the possibility to maintain the burrow in complete darkness. This apparatus is used to observe the social and sexual interactions in groups of subjects living for a long period in the environment. One of the advantages of the seminatural environment is that there are places (e.g. burrow or nest boxes) for the females to hide from the males, while at the same time being visible to the observer. The estrus statuses of the females in these studies are determined by behavioral indices instead of vaginal smears in order to disturb the subjects as little as possible. Each group remains in the environment for 8 days, and the entire 8 day period is recorded on video. Exploratory behaviors such as walking and sniffing the floor decline within 48 hours of the introduction of the subjects into the system to a low level (Chu & Ågmo, 2011).
4.2 Visible burrow system
A visible burrow system is a habitat providing burrows and an open surface area for mixed-sex rodent colonies. This approximately 1 square meter device mimics the tunnel-burrow system that laboratory rats dig for themselves when given a dirt substrate. Groups of laboratory rats in such apparatus show a variety of sociosexual activities. It is used to study sexual, defense and aggressive interactions as well as chronic social stress (e.g. Blanchard et al., 1995; Blanchard et al., 2001; Monder, Sakai, Miroff, Blanchard, & Blanchard, 1994).
4.3 Other procedures
Testing arenas incorporating elements of a representative design are also used for studying behavior in mice. A testing arena termed “semi-natural environment”, measuring 5.5 × 2.75 × 3 m high was used by Ragnauth et al. (2001; 2005). It is constructed as an open box, with 10 dark open-side plastic boxes (10 × 10 cm) and 12 transparent Plexiglas tubes (15 cm long × 6 cm diameter) randomly scattered inside of the apparatus. Nesting material is shredded into small pieces and randomly placed around, while food and water are supplied in the middle. Another smaller semi-natural environment (1.2 × 1.2 × 3 m high) was employed in Garey, Kow, Huynh, Ogawa, & Pfaff (2002). This open box device contains six acrylic “nestboxes” arranged around the periphery. Four acrylic cylinders containing nest-building material are placed in each corner at the outset of the experiment. Food and water are provided at a central location. By housing a group of female mice in these apparatus, behaviors such as nest building, herding behavior and aggressive interactions with intruders have been studied.
5. FEMALE RECEPTIVITY AND BEHAVIORAL ESTRUS
During copulation, female rats display lordosis, a distinct spinal reflex posture with flexion of the back, extension of the neck, and elevation of the hindquarters and rump. This posture usually lasts 0.5 - 1.5 s. A female displaying this behavior in response to male mounting is frequently described as sexually receptive. The period of receptivity is termed “behavioral estrus” (Long & Evans, 1922). Female rats are normally considered to be in behavioral estrus whenever they demonstrate lordosis. The duration of behavioral estrus varies between individuals. The main stimulus leading to the presentation of lordosis is tactile stimulation of the back and flanks provided by the mounting male (Kow, 1976; Kow & Pfaff, 1973; Kow, Zemlan, & Pfaff, 1980). Ovarian steroids are necessary for the display of lordosis. In the intact female, the level of estrogen regulates the length of behavioral estrus (Powers, 1970 ; Södersten & Eneroth, 1981). Ovariectomized (OVX) females never display lordosis, and estrogen treatment can restore these behaviors (e.g. Boling & Blandau, 1939; Meyerson, 1964). The level and frequency of lordosis can be used as an index to measure the status of female sexual receptivity. Moreover, the lordosis quotient (LQ), calculated by dividing the number of lordosis displayed by the number of mounts received multiplied by 100, is also used to quantify female responding to male mounting.
Receptivity has frequently been studied in the standard setting when a female rat is simply introduced to a sexually active male. For instance, an analysis of female receptivity performed by Madlafousek & Hliňák (1977) used behavioral data from 6 intact females observed in a small arena housing a sexually active male. In such setting, a gradual onset of sexual receptivity was reported, in the sense that only a fraction of the male’s mounts activated lordosis at the beginning of the behavioral estrus. This was also the case at the end of the period of sexual receptivity. Only for a few hours in the middle of estrus the female responds with lordosis to all male mounts. This coincides with results from experiments using the ovariectomized female rats, in which the reaction to estrogen treatment is dose dependent (e.g. Spiteri & Ågmo, 2006). At low doses the female displays lordosis in response to a small proportion of mounts and rejection behavior could be repeatedly demonstrated to the mounting male. With increasing dosage, the proportion of mounts with corresponding lordosis increases and the frequency of rejections is reduced. Eventually the female will display lordosis to every mount.
Based on the behavioral data, the onset and offset of female receptivity in a seminatural environment follows a different pattern than that described in a pair setting. When the sociosexual activities of a group of rats (each group consistent of 3 males and 4 intact females) were continuously observed, we noticed that the females consistently display lordosis in response to every male mount from the start of behavioral estrus until the end of it (Chu & Ågmo, 2014). This means that the female suddenly changes from a state of complete non-receptivity to a state of full receptivity and then abruptly changes back to non-receptivity. This observation coincides with the description made by Calhoun (1962a) when observing a group of wild rats that copulated in nature.
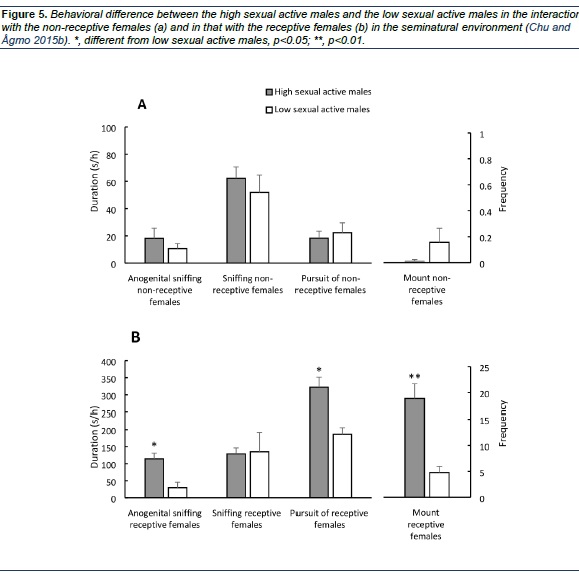
We suggested that the gradual change in receptivity described earlier is an artifact of the observation procedure. There are two possible reasons of observing the gradual change. First, the limited space in a standard setting makes it difficult for the female to avoid male pursuit. Particularly the male partner is usually selected for high sexual activity assuming that they mount non-receptive females or females that are only partially receptive. This is not the cases in a seminatural environment. Actually, in such a big and complex device, males do not pursue or mount females that are not receptive (Chu & Ågmo, 2014, 2015a). Since the males are not attracted to and do not mount females until they are fully receptive, the female transition from non-receptivity to receptivity appears to be abrupt.
This is different from what is observed in a standard observation cage, when the males frequently pursue and mount non-receptive females. One reason for this difference could be that in most standard tests males have been preselected for consistent and often high, sexual activity. Because of the preselection, the males are also sexually experience when tested. In the seminatural environment, no such selection was made. It could, then, be argued that highly active males would not distinguish between receptive and non-receptive females in the way our males do. To evaluate this proposal, we selected the 5 males with highest sexual activity and compared them to the 5 males with lowest activity in the Chu and Ågmo (2015b) study. As shown in Figure 5, the amount of pursuit of the non-receptive females and the amount of mounting the non-receptive females are similar in males with high sexual activity and in low active males. A second reason for the sudden transition from non-receptivity to receptivity could be that hormone-treated OVX females respond to hormone treatment with varying delays due to individual differences (Powers & Valenstein, 1972). Because of this, the appearance of receptivity is usually described as gradual, even though it is not when individual rather than group data are analyzed (e.g. Green, Luttge, & Whalen, 1970 ; Whalen, 1974). The sudden behavioral change in female sexual receptivity occurs also in OVX females treated with estradiol and progesterone and observed in the seminatural environment (Le Moëne, Snoeren, Chu, & Ågmo, 2015). Thus, in an externally valid procedure, the beginning and end of receptivity are entirely different from what has been observed in standard procedures.
6. FEMALE PARACOPULATORY BEHAVIORS AND ITS ROLE IN COPULATION
During sexual interaction, the female displays a series of stereotypied motor activities including ear wiggling, running, and darting. The behavior patterns have been termed “proceptive” by Beach (1976), who defined them as ‘appetitive activities shown by females in response to stimuli received from males’ (p. 114), This behavior was also named “precopulatory” (e.g. Madlafousek & Hliňák, 1977) or “female solicitation behavior” (e.g. McClintock & Adler, 1978). The complexity of the behaviors has been studied in detail by Erskine (1989). In that review, these female behaviors were further divided into several smaller categories, some of which appeared to be rare occurrences. Despite the different interpretations of the function of ear wiggling, running, hopping and darting, these behaviors are exhibited by females during mating and they are highly dependent on ovarian hormones. Female rats present no such behavior in the absence of these steroids (reviewed in Pfaff, 1980 ; Pfaff, 1999).
It has been suggested that the behavior patterns lumped together under the terms proceptive behavior, precopulatory behavior or solicitation should be labeled “paracopulatory behavior” because they are displayed during copulation, and the term does not refer to inferred but unproved functions (e.g. Blaustein, 2002, 2009). We have used that term in recent publications and it will be used in the present review.A female approaching a male or staying in close proximity to a male increases the possibility of sexual interactions. Therefore, displays of paracopulatory behavior seldom occur when a male is at a large distance from the female. Paracopulatory behavior is intensely activated by tactile stimulation provided by the male (Ågmo, 2007 ; Ågmo, Turi, Ellingsen, & Kaspersen, 2004). However, tactile stimulation is not necessary for the manifestation of paracopulatory behavior. Since these behavior patterns occur repetitively prior to and between female lordotic responding to male mounts, it is regarded as one of the behaviors associated with female’s sexual motivation (e.g. Ellingsen & Ågmo, 2004 ; Sanchez Montoya, Hernandez, Barreto-Estrada, Ortiz, & Jorge, 2010; Santoru, Berretti, Locci, Porcu, & Concas, 2014). Female paracopulatory behavior may also enhance male sexual motivation.
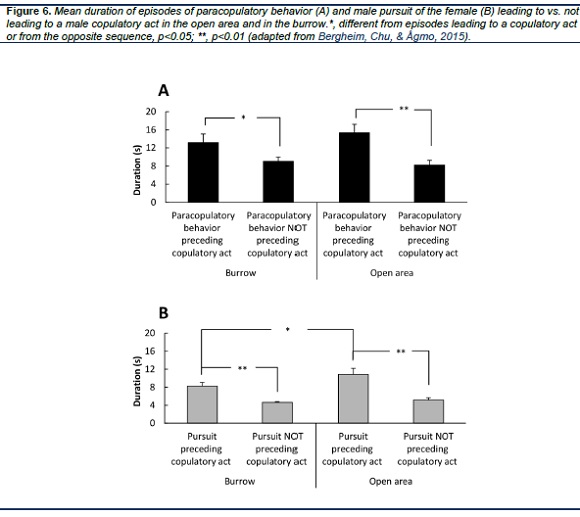
In the studies conducted with non-representative designs, the amount of paracopulatory behavior displayed by the female is reported to be a critical determinant of the likelihood that the male would initiate copulation. However, this only applies in nonresponsive, sexually inexperienced, and recently castrated males, as well as to males in long-term castration given low doses of testosterone (Hlinak & Madlafousek, 1977; Hlinak, Madlafousek, & Mohapelova, 1979; Madlafousek & Hlinák, 1983; Madlafousek, Hlinak, & Beran, 1976; Whishaw & Kolb, 1985). Lack of sexual experience is only a one-time event, and castration produces an unphysiological state. In sexually experienced rats, no relationship is found between the amount of female paracopulatory behavior and the intensity of male sexual activity (Landau & Madden, 1983). In the seminatural environment, paracopulatory behavior is strongly associated with male copulatory activities. Actually, the duration of female paracopulatory behavior is highly correlated with the amount of male mounting (r = 0.864, see Chu & Ågmo, 2015b), and modestly correlated with the duration of male pursuit of the female (r = 0.574, Chu & Ågmo, 2014). However, we found no relationship between paracopulatory behavior and the number of intromissions and ejaculations received by the females. We conducted a detailed analysis by studying a total of 5370 episodes of paracopulatory behavior and the male behavior preceding or following it (Bergheim, Chu, & Ågmo, 2015). Most episodes of paracopulatory behaviors were unrelated to male copulatory acts, since only 34 % of episodes of paracopulatory behaviors preceded a male copulatory act. However, 96% of copulatory acts occurred within 5 s of female paracopulatory behavior. Furthermore, as shown in Figure 6 A, long episodes of paracopulatory behavior were far more likely to be followed by a male copulatory act than short episodes. The dependency on time suggests that paracopulatory behaviors increase the male’s motivation to engage in sexual activities. The longer the display of paracopulatory behavior, the more increases male motivation. In addition, another remarkable result found in our studies is that at the beginning of behavioral estrus, the duration of paracopulatory behaviors suddenly increased from close to zero shortly before the first lordosis to a rather high level immediately before it (Chu & Ågmo, 2015a). This abrupt change happened within 30 seconds, thereafter, the females maintained a high level of paracopulatory behavior throughout the entire behavioral estrus (Chu & Ågmo, 2014). The opposite change appears at the end of behavioral estrus. There is a sudden reduction to zero of paracopulatory behavior after the last lordosis (Chu & Ågmo, 2015a). Actually, in females, apart from receptivity itself, paracopulatory behavior is the only activity associated with changes between receptivity and non-receptivity.
7. FEMALE SOCIAL INTERACTIONS DURING COPULATION
During mating, a female rat displays several social behaviors to the male. The affiliative physical contact often involves sniffing of the male rat’s body part and investigating the male’s anogenital region. Another affiliative behavior, which is characterized by a female running closely behind a male while both are running around the testing apparatus, but without darting and ear wiggling, is termed as “female pursuit of the male”. Female agonistic and avoiding behaviors include fleeing, nose off and rejection, has been observed during the interactions between a nonreceptive or not fully receptive female and a vigorous male (e.g. Ball, 1937; Hemmingsen, 1933; Kuehn & Beach, 1963 ; Stone, 1922). A sexually receptive female can also display such nonaffiliative behaviors during sexual interaction. For instance, the female may occasionally flee from a male, or display a nose off posture (female facing male rat either standing on 4 legs or while rearing with boxing and teeth showing). In addition to fleeing from the male and nose off to male, a female may reject the male with kicks, bites or turning around against its suitor.
It is useful to record these behaviors during copulation, particularly in studies of hormone action (e.g. Musatov, Chen, Pfaff, Kaplitt, & Ogawa, 2006; Spiteri et al., 2010; Spiteri & Ågmo, 2006) and the effects of brain lesions (e.g. Pfeifle & Edwards, 1983 ; Rivas & Mir, 1990). However, almost no systematic observation of female nonsexual behaviors during behavioral estrus has been reported in a pair setting. The results from tests in a seminatural environment (Chu & Ågmo, 2014) show that female pursuit of the male and female anogenitally sniffing male are rather infrequent during female behavioral estrus. Compared with these two interactions, female sniffing of the male occurs in a slightly bigger amount; and females sniff more at the beginning of behavioral estrus (about 3 s/min) than at the end (about 1.5 s/min). The incidence of female fleeing from the male is as low as 0.035 times per minute. Nose off shows a similar pattern. During the onset and offset of behavioral estrus, the duration or frequency of the affiliative behavior of sniffing and the nonaffiliative behaviors of nose-off remain unchanged (Chu & Ågmo, 2015a).
Rejection had a high frequency in the beginning of estrus and then declined to a constant, low level. A surprising observation in the seminatural environment was that the females failed to show any increase in rejections or other nonaffiliative behaviors towards the end of estrus. Interestingly the frequency of rejection does not change at the onset and offset of behavioral estrus (Chu & Ågmo, 2015a), that is females reject males at the same level before the beginning of estrus, at the beginning and the end of estrus, and after the end of estrus. Thus, female sexual behaviors, lordosis and paracopulatory behaviors, seem to be rather independent from other social behaviors, like sniffing and anogenital sniffing, as well as from nonaffiliative behaviors like rejections and nose off. Actually, females performing a high number of lordoses sniff the males, and are sniffed by the males, no more than females performing a low number of lordoses and they show no less nose offs or rejections (Chu & Ågmo, 2014).
There are many observations suggesting that female non-sexual behaviors are controlled differently from receptivity and paracopulatory behavior. For instance, the independence of sexual behaviors from other social behaviors has been reported by Ågmo & Soria (1997) in female rats observed in a bilevel chamber. In that study, a male rat was placed on the lower level, which allowed the female to pace sexual interactions. The latency to descend to the male’s level, the number of approaches to the male as well as the time spent on the upper level, out of reach of the male, and interactions such as sniffing, rearing, anogential sniffing were registered, and none of these activities affect female sexual behavior. Other examples are found in lesion studies. Female receptivity can be eliminated without affecting the time she spent with a sexually active male or a castrated one (Pfeifle & Edwards, 1983) while that time can be affected without reducing receptivity (Rivas & Mir, 1990). Results from different types of testing arenas reveal that female social, non-sexual activities play no role in the regulation of female sexual behavior.
8. MALE COPULATORY BEHAVIORS
Male rat’s sexual behavior consists of a highly ordered sequence of copulatory acts, mount, intromission and ejaculation. The pattern studied in a standard copulation cage is described below. Normally males would start copulating by performing a mount with or without vaginal penetration within 1-2 min following the introduction of a sexually receptive female (e.g. Chu et al., 2008; Dewsbury, 1967; Ågmo, 1997). The actual duration of each copulatory act (mount or intromission) can be rather short, lasting about 400 ms (Moralí et al., 2003). A number of intromissions, but not mounts, is necessary for a male to achieve ejaculation (Beach & Jordan, 1956). After each intromission, the male rat takes a short rest before he resumes copulation. Mounts and intromissions are performed alternately for 4 -10 min until ejaculation occurs (Ågmo, 1997). During ejaculation, the penile insertion lasts longer than of an intromission, approximately 1.5 s (Moralí et al., 2003) and is associated with rhythmic abdominal contractions. Dismount following ejaculation is rather slow and associated with an open arm posture (Lucio, Manzo, Martinez-Gomez, Sachs, & Pacheco, 1994). Since rats are multiple ejaculators, copulation is resumed after 4 - 7 min of rest (postejaculatory interval) following each ejaculation.
Male rat sexual behaviors are usually analyzed in terms of copulatory series in non-representative designs. A copulatory series is defined as beginning with a mount or intromission and ending in an ejaculation, since rats are multiple ejaculators, copulation is resumed within a few minutes of ejaculation. In very long tests, a state of sexual exhaustion, or satiety, is reached. This state is normally defined as a male’s failure to resume copulation within 30 min or 1 h of the prior ejaculation (e.g. Ågmo, 1999). The behavioral parameters, including numbers and latencies of copulatory acts (mount, intromission and ejaculation) are organized by copulatory series.
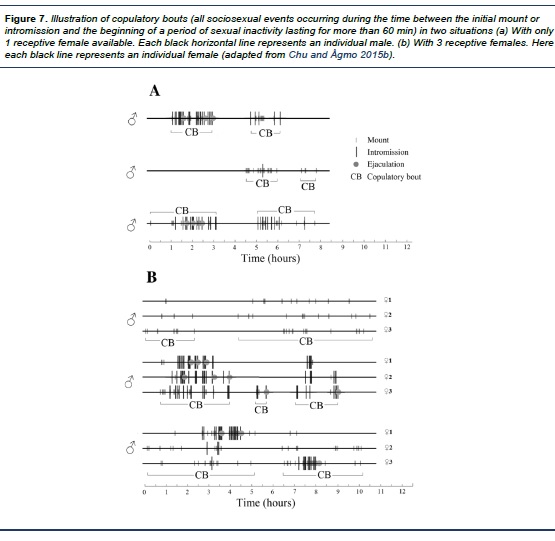
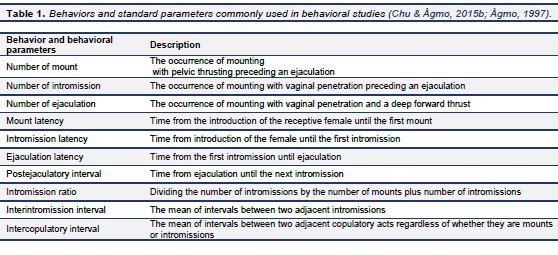
In the seminatural environment, the males’ behavior is different. In this case, a long period of sexual inactivity is frequently preceded not by an ejaculation but by a mount or an intromission (Chu & Ågmo, 2015b). Thus, ejaculation does not appear to be a typical end point. Instead of using copulatory series, male sexual behaviors are arranged in units of “copulatory bouts”, defined as the time between the initial mount or intromission and the beginning of a period of sexual inactivity lasting for more than 60 min. This criterion has earlier been used for defining mammalian copulatory patterns (Dewsbury & Pierce, 1989). Examples of copulatory bouts are shown in Figure 7. A series of frequent sexual interactions occurred within each bout, which could consist of several copulatory series or just a few mounts. Organizing male sexual behaviors in copulatory bouts provides more useful information than the series by including incomplete series and excluding long segments without sociosexual activity.In order to generate a quantified description of male sexual activity, relevant behaviors and some of the standard parameters listed in Table 1 are usually registered (Chu & Ågmo, 2015b ; Ågmo, 1997). When male rats are allowed to perform several copulatory series, there is a decline in the number of preejaculatory intromissions as well as of the ejaculation latency from the first to subsequent ejaculations while there is a progressive increase in the postejaculatory interval (e.g. Dewsbury, 1967; Larsson, 1956). Male sexual behavior in the seminatural environment follows a comparable pattern, changes in the number of preejaculatory intromissions (Chu & Ågmo, 2015b).
The traditional way of describing male sexual behavior as a series of ejaculations in which the last ejaculation is followed by a long period of sexual inactivity turned out to be insufficient. Instead, in a seminatural environment, male copulatory behaviors more likely occur in bouts. Actually, the analysis of male sexual behavior in terms of copulatory bouts have been found useful in prolonged observations of sexual behavior in several other mammalian species (Carter & Getz, 1985; Carter, Getz, & Cohen-Parsons, 1986; Savage-Rumbaugh & Wilkerson, 1978; Whalen, 1963).There could be one or several copulatory bouts during the long observation in a seminatural environment (Chu & Ågmo, 2015b). The length of a bout is unrelated to the intensity of male sexual behaviors (e.g. mount and intromission) but is associated with the total amount of those behaviors. The distribution of male copulatory events are quite stable throughout a bout (see Figure 7), as there is no increase in interintromission interval or intercopulatory interval at the end of a bout. The reasons for males ceasing to copulate at the end of a bout may not involve sexual fatigue or exhaustion. An exhausted rat needs several days of rest before expressing copulatory behavior at the same level as before exhaustion (Beach & Jordan, 1956; Larsson, 1956; Rodríguez-Manzo & Fernández-Guasti, 1994). However, the males housed in a seminatural environment resumed copulation with undiminished intensity after a mean resting period of 21.2 hours (based on data from Chu & Ågmo, 2015b).In addition, there is a quantitative difference in male copulatory activity. In the standard test in a small cage, about 7 - 9 ejaculations are achieved before exhaustion (Beach & Jordan, 1956 ; Larsson, 1956; Rodríguez-Manzo & Fernández-Guasti, 1994; Tiefer, 1969). This number contrasts dramatically to the 3 - 4 ejaculations observed in the seminatural environment, in which, the males have continuous access to the receptive females during the entire period of estrus (mean duration is 7.4 hours, Chu & Ågmo, 2014) and. In a small testing arena, it was reported that male rats could copulate with females that are not fully receptive (e.g. Madlafousek & Hliňák, 1977 ; Spiteri & Ågmo, 2006). In the seminatural environment, male rats show very low sexual interest in such females. Actually, in a group of rats, copulatory action is hardly spotted when females are outside of their behavioral estrus; it appears that males only copulates with females in the state of full receptivity (Chu & Ågmo, 2015a , 2015b). When there are several females in behavioral estrus, male rats copulate in promiscuous patterns, which means that they mount on any of these females without showing copulatory preference (Chu & Ågmo, 2015b). Likewise, females copulate with all males in a completely random pattern (Chu & Ågmo, 2014).
9. MALE SOCIAL INTERACTIONS DURING COPULATION
The analyses of male social behaviors provide useful information about the animals’ general arousal and make it possible to determine if social interactions affect sexual behavior specifically. In a small copulation cage, the intensity of investigatory behavior, male sniffing female and anogenital sniffing female, shows no consistent association with male copulatory behavior (Giordano, Güemes, López-Arias, & Paredes, 1998; Paredes, Highland, & Karam, 1993). On the contrary, pursuit of the female appears to be a good indicator of male readiness to copulate (e.g. Dewsbury, 1967 ; Giordano et al., 1998; Paredes et al., 1993).
In the studies using the seminatural environment, males and females were housed together for 8 days and continuously observed. This is different from the short observations in the conventional testing arenas, and this difference in duration of observation allowed us to find several important results. As reported by Chu & Ågmo (2015b), male rats use 77 % of their time resting, wandering, or other activities without interaction with another subject while they are sexually active (within a copulatory bout). This “self-entertaining” behavior accounted for the greatest proportion of time of a copulatory bout. Males only used about 12% of this time to pursue (8%) and sniff (4%) receptive females. Outside of copulatory bouts, there is very little nonsexual social interaction. In fact, over 90% of social behaviors occurred during copulatory bouts. Between bouts, the main social activity is sniffing female rats. In addition, males hardly provoke any fights; the most aggressive behavior observed is nose off. There is indeed an avoidance action between males that is described as a male fleeing or running away from another male. However, fleeing is very uncommon. This coincides with the observations that competition for sex between males is unusual in the wild (Barnett, 1958, 1975) and that wild rats usually live peacefully together in large packs (Eibl-Eibesfeldt, 1961) with attacks only occurring towards unfamiliar introducers. Nevertheless, it may be assumed that antisocial behavior depends on population density, and that the density in the seminatural environment was too low to activate such behavior. However, population density in wild rats is much lower than in our environment. A rough estimate based on the data provided in Davis, Emlen, & Stokes (1948) show that the density of wild rats is 0.02 rats per m2, which is much lower than that in the seminatural environment (1.25 rats per m2). Even though wild rat population density can vary remarkably even over short geographical distances, it does not reach the level of the seminatural environment (Himsworth, Jardine, Parsons, Feng, & Patrick, 2014). This means that even in a context with high population density, a group of rats rarely displays aggressive interactions. Population densities far above those used in the studies mentioned herein may, of course, be associated with frequent antisocial behaviors (Calhoun, 1962b).
Males only pursue fully receptive females (Chu & Ågmo, 2015a). The amount of male pursuit of female is strongly correlated with the number of mounts (Chu & Ågmo, 2014 , 2015b). Male pursuit of the female precedes most sexual interactions and males initiate sexual interaction as often as females. Moreover, the duration of an episode of male pursuit of the female is an important determinant of the likelihood that a copulatory act will follow (Bergheim et al., 2015). Apart from this, male sexual behaviors, intromission and ejaculation, seem to be independent of affiliative behaviors, like sniffing and anogenital sniffing, as well as from nonaffiliative behaviors like fight and nose off. This independence of sexual behaviors from other social behaviors coincides with that mentioned earlier for male rats observed in smaller testing apparatus.
10. COMPARISON OF SEXUAL BEHAVIOR IN HUMAN AND RATS
Polygamous mating structure is estimated to occur in up to 90% of mammalian species (Aloise King, Banks, & Brooks, 2013). Anthropological studies of human societies have released that about 90% of them practice polygyny, whereas only 3% are polyandrous. Remaining societies are monogamous, at least socially monogamous. Promiscuity seems to be uncommon among humans, at least as a socially acceptable mating strategy. There are many reasons to be believe that human sexual behavior in most aspects is a social construction. The main determinant of with whom, when, where and how we copulate is dominant social norm. However, as shown in the brilliant studies of Kinsey and collaborators (Kinsey, Pomeroy, & Martin, 1948), many humans escape from the social norm and engage in sexual activities that are not only contradictory to the norm, but also illegal. Presently, there are groups in many places that overtly or covertly reject the traditional morality rules about sex. This is the case, among many others, among the men and women, who frequent sex clubs. Actually there are reports from group-sex events, particularly involving gay men (Meunier, 2014 ; Tewksbury, 2002), but also from heterosexual groups (Friedman et al., 2008), suggesting that different copulatory acts are performed with several partners simultaneously. This pattern is highly comparable to that of male rats during sexual interaction with several receptive females. The males seem to copulate with different females in a completely haphazard way. Female rats also receive mounts, intromissions and ejaculations from several males in what appears to be a rather disordered way. It is quite possible that a promiscuous mating strategy would be chosen by many humans if societal norms would be less discouraging for that choice. Even though there is no experimental evidence for this notion, it is generally accepted that human sexual behavior is socially constructed (Foucault, 1984 ; Gagnon and Simon, 2002). Consequently, social norms are the main determinant of how this behavior is expressed. As mentioned, humans on the side of mainstream norms may be exactly as promiscuous as our rats (see also O'Byrne and Watts, 2011). Perhaps we have more in common with rodents than we would like to accept.
In the human male, sexual activity normally ends at ejaculation. Multiple ejaculations are quite infrequent. Furthermore, the biological function of sex, the transfer of gametes, is completed with the ejaculation. The purpose of the behavior is thereby fulfilled. In rats, however, ejaculation is not the end point of sexual behavior. The overwhelming majority of rats will display multiple ejaculations in rapid succession before ceasing to copulate (see Ågmo, 1997), and when they do so the last copulatory act may be either mount, intromission or ejaculation (Chu & Ågmo, 2015b). Thus, different from human, ejaculation is not more likely to end a behavioral sequence than any other of the copulatory acts in rats.
11. CONCLUSIONS
It would be reasonable to assume that any research regarding animal social and sexual behavior could profit from the procedures used in the study. The seminatural environment described here seems to be an obvious candidate for providing useful information that could be generalized to other contexts. The standard copulation cage has proved its respectable value, but due to the lack of external validity and its highly artificial design, it may lead to spurious or contradictory results.
Studies of sociosexual behaviors in a seminatural environment provide a substantial amount of information that are not possible be obtain in a pair test in a small cage. Likewise, long observation periods allow for an analysis of the structure of female and male sexual behavior that is impossible to discover in time-limited tests. Results from this kind of study can generate meaningful knowledge about the adaptive value of the many features of sociosexual interactions. We suggest that the seminatural environment employed here not only is a good example of a representative design but also that it is needed for a serious evaluation of the many speculations concerning the functional importance of behavioral items.
12. REFERENCES
Ågmo, A. (1997). Male rat sexual behavior. Brain Research Protocols, 1(2), 203-209. [ Links ]
Ågmo, A. (1999). Sexual motivation-an inquiry into events determining the occurrence of sexual behavior. Behavioural Brain Research, 105(1), 129-150. doi: http://dx.doi.org/10.1016/S0166-4328(99)00088-1. [ Links ]
Ågmo, A. (2003). Unconditioned sexual incentive motivation in the male Norway rat (Rattus norvegicus). Journal of Comparative Psychology, 117(1), 3-14. [ Links ]
Ågmo, A. (2007). Functional and dysfunctional sexual behavior a synthesis of neuroscience and comparative psychology. United Kingdom: Academic Press. [ Links ]
Ågmo, A., & Soria, P. (1997). GABAergic drugs and sexual motivation, receptivity and exploratory behaviors in the female rat. Psychopharmacology (Berl), 129(4), 372-381. [ Links ]
Ågmo, A., Turi, A. L., Ellingsen, E., & Kaspersen, H. (2004). Preclinical models of sexual desire: conceptual and behavioral analyses. Pharmacology Biochemistry and Behavior,78(3), 379-404. doi: 10.1016/j.pbb.2004.04.013. [ Links ]
Aloise King, E. D., Banks, P. B., & Brooks, R. C. (2013). Sexual conflict in mammals: Consequences for mating systems and life history. Mammal Review, 43(1), 47-58. [ Links ]
Ball, J. (1937). A test for measuring sexual excitability in the female rat: Johns Hopkins Press. [ Links ]
Barnett, S. A. (1958). An analysis of social behaviour in wild rats. Proceedings of the Zoological Society of London, 130(1), 107-152. [ Links ]
Barnett, S. A. (1975). The rat: a study in behavior. Chicago: University of Chicago Press. [ Links ]
Beach, F. A. (1976). Sexual attractivity, proceptivity, and receptivity in female mammals. Hormones and Behavior, 7(1), 105-138. doi: Doi 10.1016/0018-506x(76)90008-8. [ Links ]
Barto, L., & Trojan, S. (1982). Male rat ejaculatory potential in a multiple-female situation in the course of four consecutive days. Behavioral and Neural Biology, 34(4), 411-420. doi: http://dx.doi.org/10.1016/S0163-1047(82)91828-3. [ Links ]
Beach, F. A., & Jordan, L. (1956). Sexual exhaustion and recovery in the male rat. Quarterly Journal of Experimental Psychology, 8(3), 121-133. doi: 10.1080/17470215608416811. [ Links ]
Bergheim, D., Chu, X., & Ågmo, A. (2015). The function and meaning of female rat paracopulatory (proceptive) behaviors. Behavioural Processes, 118, 34-41. doi: 10.1016/j.beproc.2015.05.011. [ Links ]
Blanchard, D. C., Spencer, R. L., Weiss, S. M., Blanchard, R. J., McEwen, B., & Sakai, R. R. (1995). Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology, 20(2), 117-134. [ Links ]
Blanchard, R. J., Dulloog, L., Markham, C., Nishimura, O., Nikulina Compton, J., Jun, A., ... Blanchard, D. C. (2001). Sexual and aggressive interactions in a visible burrow system with provisioned burrows. Physiology & Behavior, 72(1-2), 245-254. [ Links ]
Blaustein, J. D. (2002). Feminine Sexual Behavior: Cellular Integration of Hormonal and Aflerent Information in the Rodent Forebrain. Hormones, Brain and Behavior, Five-Volume Set. [ Links ]
Blaustein, J. D. (2009). Feminine reproductive behavior and physiology in rodents: integration of hormonal, behavioral, and environmental influences. In D. W. Pfaff, A. P. Arnold, S. E. Fahrbach, A. M. Etgen & R. T. Rubin (Eds.), Hormones, Brain and Behavior (Second Edition) (pp. 67-108). San Diego: Academic Press. [ Links ]
Boling, J. L., & Blandau, R. J. (1939). The estrogen-progesterone induction of mating responses in the spayed female rat. Endocrinology,25(3), 359-364. doi: doi:10.1210/endo-25-3-359. [ Links ]
Brunswik, E. (1955). Representative design and probabilistic theory in a functional psychology. Psychological Review, 62(3), 193-217. doi: Doi 10.1037/H0047470. [ Links ]
Brunswik, E., & Kamiya, J. (1953). Ecological cue-validity of proximity and of other Gestalt factors. The American Journal of Psychology, 66(1), 20-32. [ Links ]
Calhoun, J. B. (1962a). The ecology and sociology of the Norway rat. Washington, D.C.: US Governnment Printing Office. [ Links ]
Calhoun, J. B. (1962b). Population density and social pathology. Scientific American, 206, 139-150. [ Links ]
Carter, C. S., & Getz, L. L. (1985). Social and hormonal determinants of reproductive patterns in the prairie vole. In Neurobiology (pp. 18-36). Berlin Heidelberg: Springer. [ Links ]
Carter, C. S., Getz, L. L., & Cohen-Parsons, M. (1986). Relationships between social organization and behavioral endocrinology in a monogamous mammal. In: J. S. Rosenblatt, C. Beer, M. Busnel and P. J. B. Slater (Eds.), Advances in the Study of Behavior (Vol. Volume 16, pp. 109-145): Academic Press. [ Links ]
Chu, X., & Ågmo, A. (2008). Sexual incentive motivation in old male rats: The effects of sildenafil and a compound (Impaza) stimulating endothelial NO synthase. Pharmacology, Biochemistry and Behavior, 89, 209-217. doi: http://dx.doi.org10.1016/j.pbb.2007.12.012. [ Links ]
Chu, X., Zhavbert, E. S., Dugina, J. L., Kheyfets, I. A., Sergeeva, S. A., Epstein, O. I., & Ågmo, A. (2008). Sildenafil and a compound stimulating endothelial NO synthase modify sexual incentive motivation and copulatory behavior in male Wistar and Fisher 344 rats. Journal of Sexual Medicine, 5(9), 2085-2099. doi: DOI 10.1111/j.1743-6109.2008.00937.x. [ Links ]
Chu, X., & Ågmo, A. (2011). Sociosexual interaction among intact male and female rats in a seminatural environment. Paper presented at the Annual meeting in Neuroscience 2011, Washington D.C. [ Links ]
Chu, X., & Ågmo, A. (2014). Sociosexual behaviours in cycling, intact female rats (Rattus norvegicus) housed in a seminatural environment. Behaviour, 151(8), 1143-1184. doi: 10.1163/1568539x-00003177. [ Links ]
Chu, X., & Ågmo, A. (2015a). Sociosexual behaviors during the transition from non-receptivity to receptivity in rats housed in a seminatural environment. Behavioural Processes, 113(0), 24-34. doi: http://dx.doi.org/10.1016/j.beproc.2015.01.001. [ Links ]
Chu, X., & Ågmo, A. (2015b). Sociosexual behaviors of male rats (Rattus norvegicus) in a seminatural environment. Journal of Comparative Psychology, 129(2), 132-144. doi: 10.1037/a0038722. [ Links ]
Chu, X., & Ågmo, A. (2016). The adrenergic α2-receptor, sexual incentive motivation and copulatory behavior in the male rat. Pharmacology Biochemistry and Behavior, 144, 33-44. doi: http://dx.doi.org/10.1016/j.pbb.2016.02.008. [ Links ]
Davis, D. E., Emlen, J. T., & Stokes, A. W. (1948). Studies on Home Range in the Brown Rat. Journal of Mammalogy, 29(3), 207-225. doi:10.2307/1375387. [ Links ]
Dewsbury, D. A. (1967). A quantitative description of the behavior of rats during copulation. Behaviour,29(2/4), 154-178. doi: 10.2307/4533188. [ Links ]
Dewsbury, D. A., & Pierce, J. D. (1989). Copulatory patterns of primates as viewed in broad mammalian perspective. American Journal of Primatology, 17(1), 51-72. [ Links ]
Eibl-Eibesfeldt, I. (1961). The fighting behavior of animals. Scientific American, 205, 112-122. [ Links ]
Ellingsen, E., & Ågmo, A. (2004). Sexual-incentive motivation and paced sexual behavior in female rats after treatment with drugs modifying dopaminergic neurotransmission. Pharmacology Biochemistry and Behavior, 77(3), 431-445. doi: http://dx.doi.org/10.1016/j.pbb.2003.12.008. [ Links ]
Erskine, M. S. (1985). Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behavioral Neuroscience, 99(1), 151-161. doi: 10.1037/0735-7044.99.1.151. [ Links ]
Erskine, M. S. (1989). Solicitation behavior in the estrous female rat: a review. Hormones and Behavior, 23(4), 473-502. doi: 10.1016/0018-506x(89)90037-8. [ Links ]
Foucault, M. (1984). Histoire de la sexualité. II. L'usage des plaisirs. Paris: Gallimard. [ Links ]
Friedman, S. R., Bolyard, M., Khan, M., Maslow, C., Sandoval, M., Mateu-Gelabert, P., Aral, S. O. (2008). Group sex events and HIV/STI risk in an urban network. Jaids-Journal of Acquired Immune Deficiency Syndromes, 49(4), 440-446. doi: 10.1097/QAI.0b013e3181893f31. [ Links ]
Gagnon, J. H., Simon, W. (2002). Sexual conduct: the social sources of human sexuality, 2nd ed. New Brunswick, NJ: Aldine Transaction. [ Links ]
Garey, J., Kow, L. M., Huynh, W., Ogawa, S., & Pfaff, D. W. (2002). Temporal and spatial quantitation of nesting and mating behaviors among mice housed in a semi-natural environment. Hormones and Behavior, 42(3), 294-306. doi: http://dx.doi.org/10.1006/hbeh.2002.1823. [ Links ]
Giordano, M., Güemes, M., López-Arias, V., & Paredes, R. G. (1998). Socio-sexual behavior in male rats after lesions of the dorsolateral tegmentum. Physiology & Behavior, 65(1), 89-94. doi: http://dx.doi.org/10.1016/S0031-9384(98)00146-2. [ Links ]
Green, R., Luttge, W. G., & Whalen, R. E. (1970). Induction of receptivity in ovariectomized female rats by a single intravenous injection of estradiol-17β. Physiology & Behavior, 5(2), 137-141. doi: 10.1016/0031-9384(70)90056-9. [ Links ]
Hemmingsen, A. M. (1933). Studies on the oestrus-producing hormone (oestrin). Skandinavisches Archiv Für Psysiologie, 65 (1), 97-250. doi: 10.1111/j.1748-1716.1933.tb00348.x. [ Links ]
Himsworth, C. G., Jardine, C. M., Parsons, K. L., Feng, A. Y. T., & Patrick, D. M. (2014). The characteristics of wild rat (rattus spp.) populations from an inner-city neighborhood with a focus on factors critical to the understanding of rat-associated zoonoses. PLoS One, 9(3), e91654. doi:10.1371/journal.pone.0091654. [ Links ]
Hlinak, Z., & Madlafousek, J. (1977). Female precopulatory behaviour as a determinant of sexual activity in male rats [proceedings]. Activitas Nervosa Superior (Praha), 19(3), 242-243. [ Links ]
Hlinak, Z., Madlafousek, J., & Mohapelova, A. (1979). Initiation of copulatory behavior in castrated male rats injected with critically adjusted doses of testosterone. Hormones and Behavior, 13(1), 9-20. [ Links ]
Kinsey, A. C., Pomeroy, W. B., & Martin, C. E. (1948). Sexual behavior in the human male. Philadelphia: Saunders. [ Links ]
Kow, L. M. (1976). Sensory requirements for the lordosis reflex in female rats. Brain Research, 101(1), 47-66. [ Links ]
Kow, L. M., & Pfaff, D. W. (1973). Effects of estrogen treatment on the size of receptive field and response threshold of pudendal nerve in the female rat. Neuroendocrinology, 13(4), 299-313. [ Links ]
Kow, L. M., Zemlan, F. P., & Pfaff, D. W. (1980). Responses of lumbosacral spinal units to mechanical stimuli related to analysis of lordosis reflex in female rats. Journal of Neurophysiology, 43(1), 27-45. [ Links ]
Kuehn, R. E., & Beach, F. A. (1963). Quantitative measurement of sexual receptivity in female rats. Behaviour, 21(3), 282-299. [ Links ]
Landau, I. T., & Madden, J. E. (1983). Hormonal regulation of female proceptivity and its influence on male sexual preference in rats. Physiology & Behavior, 31(5), 679-685. [ Links ]
Larsson, K. (1956). Conditioning and sexual behavior in the male albino rat. Stockholm: Almqvist och Wiksell. [ Links ]
Le Moëne, O., Snoeren, E., Chu, X., & Ågmo, A. (2015, October 17-21). Changes in socio-sexual interactions during transition from non-estrus to estrus in hormone-treated, ovariectomized rats housed in a semi-natural environment. Paper presented at the Annual meeting in Neuroscience 2015, Chicago. [ Links ]
Long, A., & Evans, H. M. (1922). The oestrus cycle of the rat and its associated phenomena. Memoirs of the University of California, 6, 1-148. [ Links ]
Lucio, R. A., Manzo, J., Martinez-Gomez, M., Sachs, B. D., & Pacheco, P. (1994). Participation of pelvic nerve branches in male rat copulatory behavior. Physiology & Behavior, 55(2), 241-246. [ Links ]
Madlafousek, J., & Hlinák, Z. (1983). Importance of female's precopulatory behaviour for the primary initiation of male's copulatory behaviour in the laboratory rat. Behaviour, 86(3), 237-248. doi: 10.1163/156853983X00381. [ Links ]
Madlafousek, J., & Hliňák, Z. (1977). Sexual behaviour of the female laboratory rat: inventory, patterning, and measurement. Behaviour, 63(3/4), 129-174. doi: 10.2307/4533852. [ Links ]
Madlafousek, J., Hlinak, Z., & Beran, J. (1976). Decline of sexual behavior in castrated male rats: effects of female precopulatory behavior. Hormones and Behavior, 7(2), 245-252. [ Links ]
McClintock, M. K. (1987). A functional approach to the behavioral endocrinology of rodents. In D. Crew (Ed.), Psychobiology of reproductive behavior: an evolutionary approach (pp. 176-203). Englewood Cliffs, NJ: Prentice-Hall. [ Links ]
McClintock, M. K., & Adler, N. T. (1978). The role of the female during copulation in wild and domestic norway rats (Rattus norvegicus). Behaviour, 67(1/2), 67-96. doi: 10.2307/4533921. [ Links ]
McClintock, M. K., & Anisko, J. J. (1982). Group mating among Norway rats I. Sex differences in the pattern and neuroendocrine consequences of copulation. Animal Behaviour, 30(2), 398-409. doi: http://dx.doi.org/10.1016/S0003-3472(82)80051-1. [ Links ]
McClintock, M. K., Anisko, J. J., & Adler, N. T. (1982). Group mating among Norway rats II. The social dynamics of copulation: Competition, cooperation, and mate choice. Animal Behaviour,30(2), 410-425. doi: http://dx.doi.org/10.1016/S0003-3472(82)80052-3. [ Links ]
Mendelson, S. D., & Gorzalka, B. B. (1987). An improved chamber for the observation and analysis of the sexual behavior of the female rat. Physiology & Behavior, 39(1), 67-71. doi: http://dx.doi.org/10.1016/0031-9384(87)90345-3. [ Links ]
Mendelson, S. D., & Pfaus, J. G. (1989). Level searching: a new assay of sexual motivation in the male rat. Physiology & Behavior, 45(2), 337-341. [ Links ]
Meunier, E. (2014). No attitude, no standing around: the organization of social and sexual interaction at a gay male private sex party in New York city. Archives of Sexual Behavior, 43(4), 685-695. doi: 10.1007/s10508-013-0182-1 [ Links ]
Meyerson, B. J. (1964). Central nervous monoamines and hormone induced estrus behaviour in the spayed rat. Acta physiologica Scandinavica. Supplementum., 241, 1-32. [ Links ]
Miller, S. D., Russell, J. C., MacInnes, H. E., Abdelkrim, J., & Fewster, R. M. (2010). Multiple paternity in wild populations of invasive Rattus species. New Zealand Journal of Ecology, 34(3), 360-363. [ Links ]
Monder, C., Sakai, R. R., Miroff, Y., Blanchard, D. C., & Blanchard, R. J. (1994). Reciprocal changes in plasma corticosterone and testosterone in stressed male rats maintained in a visible burrow system: evidence for a mediating role of testicular 11 beta-hydroxysteroid dehydrogenase. Endocrinology, 134(3), 1193-1198. [ Links ]
Moralí, G., Soto, M. A. P., Contreras, J. L., Arteaga, M., Gonzalez-Vidal, M. D., & Beyer, C. (2003). Detailed analysis of the male copulatory motor pattern in mammals: Hormonal bases. Scandinavian Journal of Psychology, 44(3), 279-288. doi: 10.1111/1467-9450.00346. [ Links ]
Musatov, S., Chen, W., Pfaff, D. W., Kaplitt, M. G., & Ogawa, S. (2006). RNAi-mediated silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proceedings of the National Academy of Sciences of the United States of America,103(27), 10456-10460. doi: 10.1073/pnas.0603045103. [ Links ]
O'Byrne, P., Watts, J. A., 2011. Exploring sexual networks: A pilot study of swingers' sexual behaviour and health-care-seeking practices. Canadian Journal of Nursing Research, 43, 80-97. [ Links ]
Paredes, R. G., Highland, L., & Karam, P. (1993). Socio-sexual behavior in male rats after lesions of the medial preoptic area: evidence for reduced sexual motivation. Brain Research, 618(2), 271-276. doi: http://dx.doi.org/10.1016/0006-8993(93)91275-W. [ Links ]
Petrinovich, L. (1979). Probabilistic functionalism: a conception of research method. American Psychologist, 34(5), 373-390. [ Links ]
Petrinovich, L., & Patterson, T. L. (1980). Field studies of habituation: III. Playback contingent on the response of the white-crowned sparrow. Animal Behaviour, 28(3), 742-751. doi: http://dx.doi.org/10.1016/S0003-3472(80)80134-5. [ Links ]
Pfaff, D. W. (1980). Estrogens and brain function: Neural analysis of a hormone-controlled mammalian reporductive behavior. New York: Springer-Verlag. [ Links ]
Pfaff, D. W. (1999). Drive: Neurobiological and molecular mechanisms of sexual motivation. Cambridge, Mass.: MIT Press. [ Links ]
Pfaus, J. G., Smith, W. J., & Coopersmith, C. B. (1999). Appetitive and consummatory sexual behaviors of female rats in bilevel chambers: I. A correlational and factor analysis and the effects of ovarian hormones. Hormones and Behavior, 35(3), 224-240. doi: http://dx.doi.org/10.1006/hbeh.1999.1516. [ Links ]
Pfeifle, J. K., & Edwards, D. A. (1983). Midbrain lesions eliminate sexual receptivity but spare sexual motivation in female rats. Physiology & Behavior, 31(3), 385-389. [ Links ]
Powers, J. B. (1970). Hormonal control of sexual receptivity during the estrous cycle of the rat. Physiology & Behavior, 5(8), 831-835. [ Links ]
Powers, J. B., & Valenstein, E. S. (1972). Individual differences in sexual responsiveness to estrogen and progesterone in ovariectomized rats. Physiology & Behavior, 8(4), 673-676. doi: 10.1016/0031-9384(72)90093-5 [ Links ]
Ragnauth, A., Moy, V., Brewer, C., Kow, L. M., Ogawa, S., & Pfaff, D. W. (2001). Female oxytocin gene knockout (OTKO) mice in a semi-natural environment (SNe) exhibit altered aggressive behaviors. Society for Neuroscience Abstracts, 27(2), 1981. [ Links ]
Ragnauth, A. K., Devidze, N., Moy, V., Finley, K., Goodwillie, A., Kow, L. M., ... Pfaff, D. W. (2005). Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain and Behavior, 4(4), 229-239. doi: 10.1111/j.1601-183X.2005.00118.x [ Links ]
Rivas, F. J., & Mir, D. (1990). Effects of nucleus accumbens lesion on female rat sexual receptivity and proceptivity in a partner preference paradigm. Behavioural Brain Research, 41(3), 239-249. doi: http://dx.doi.org/10.1016/0166-4328(90)90111-Q. [ Links ]
Robitaille, J. A., & Bouvet, J. (1976). Field observations on the social behaviour of the Norway rat, Rattus norvegicus (Berkenhout). Behavioral Biology, 1, 289-308. [ Links ]
Rodríguez-Manzo, G., & Fernández-Guasti, A. (1994). Reversal of sexual exhaustion by serotonergic and noradrenergic agents. Behavioural Brain Research,62(2), 127-134. doi: 10.1016/0166-4328(94)90019-1. [ Links ]
Sanchez Montoya, E. L., Hernandez, L., Barreto-Estrada, J. L., Ortiz, J. G., & Jorge, J. C. (2010). The testosterone metabolite 3alpha-diol enhances female rat sexual motivation when infused in the nucleus accumbens shell. Journal of Sexual Medicine, 7(11), 3598-3609. doi: 10.1111/j.1743-6109.2010.01937.x. [ Links ]
Santoru, F., Berretti, R., Locci, A., Porcu, P., & Concas, A. (2014). Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl), 231(17), 3351-3364. doi: 10.1007/s00213-014-3539-9. [ Links ]
Savage-Rumbaugh, E. S., & Wilkerson, B. J. (1978). Socio-sexual behavior in Pan paniscus and Pan troglodytes: A comparative study. Journal of Human Evolution, 7(4), 327-IN326. doi: http://dx.doi.org/10.1016/S0047-2484(78)80074-8. [ Links ]
Slob, A. K., de Klerk, L. W., & Brand, T. (1987). Homosexual and heterosexual partner preference in ovariectomized female rats: Effects of testosterone, estradiol and mating experience. Physiology & Behavior, 41(6), 571-576. [ Links ]
Snoeren, E. M. S., Antonio-Cabrera, E., Spiteri, T., Musatov, S., Ogawa, S., Pfaff, D. W., & Ågmo, A. (2015). Role of oestrogen α receptors in sociosexual behaviour in female rats housed in a seminatural environment. Journal of Neuroendocrinology, 27(11), 803-818. doi: 10.1111/jne.12321. [ Links ]
Snoeren, E. M. S., Lehtimaki, J., & Agmo, A. (2012). Effect of dexmedetomidine on ejaculatory behavior and sexual motivation in intact male rats. Pharmacology Biochemistry and Behavior, 103(2), 345-352. doi: 10.1016/j.pbb.2012.09.007. [ Links ]
Spiteri, T., Musatov, S., Ogawa, S., Ribeiro, A., Pfaff, D. W., & Ågmo, A. (2010). Estrogen-induced sexual incentive motivation, proceptivity and receptivity depend on a functional estrogen receptor alpha in the ventromedial nucleus of the hypothalamus but not in the amygdala. Neuroendocrinology,91(2), 142-154. doi: 10.1159/000255766. [ Links ]
Spiteri, T., Ogawa, S., Musatov, S., Pfaff, D. W., & Ågmo, A. (2012). The role of the estrogen receptor a in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behavioural Brain Research, 230(1), 11-20. doi: 10.1016/j.bbr.2012.01.048. [ Links ]
Spiteri, T., & Ågmo, A. (2006). Modèles précliniques du désir sexuel. Sexologies, 15(4), 241-249. doi: 10.1016/j.sexol.2006.05.001. [ Links ]
Stone, C. P. (1922). The congenital sexual behavior of the young male albino rat. Journal of Comparative Psychology, 2(2), 95. [ Links ]
Södersten, P., & Eneroth, P. (1981). Serum levels of oestradiol-17β and progesterone in relation to sexual receptivity in intact and ovariectomized rats. Journal of Endocrinology, 89(1), 45-54. [ Links ]
Tewksbury, R. (2002). Bathhouse intercourse: Structural and behavioral aspects of an erotic oasis. Deviant Behavior, 23(1), 75-112. doi: 10.1080/016396202317192035. [ Links ]
Tiefer, L. (1969). Copulatory behaviour of male Rattus norvegicus in a multiple-female exhaustion test. Animal Behaviour, 17(4), 718-721. doi: http://dx.doi.org/10.1016/S0003-3472(69)80018-7. [ Links ]
Whalen, R. E. (1963). Sexual Behavior of Cats. Behaviour, 20(3/4), 321-342. [ Links ]
Whalen, R. E. (1974). Estrogen-progesterone induction of mating in female rats. Hormones and Behavior,5(2), 157-162. doi: http://dx.doi.org/10.1016/0018-506X(74)90040-3. [ Links ]
Whishaw, I. Q., & Kolb, B. (1985). The mating movements of male decorticate rats: evidence for subcortically generated movements by the male but regulation of approaches by the female. Behavioural Brain Research, 17(3), 171-191. [ Links ]