1. Introduction
Autosomal Dominant Cerebral Arteriopathy with Sub cortical Infarcts and Leukoencephalopathy (CADASIL) was first cited in 1991 by TournierLasserve, IbaZizen, Romero, and Bousser (1991), who described the disease as having an autosomal dominant pattern based on the index case of a 50yearold man with aphasia and severe headache (nausea and photophobia) who developed pseu dobulbar palsy, dysarthria and the inability to walk as well as presenting depression and apathy before finally developing dementia. After investigation, the authors were able to associate these phenotypic traits to similar cases found in Spain since 1970, and named the disease using the acronym CADASIL (López & Vilanova, 2009; TournierLasserve et al., 1991).
CADASIL is a type of multiinfarct vascular demen tia caused by mutations in the NOTCH3 (Neurogenic locus notch homolog protein 3) gene, located in the short arm of chromosome 19. This gene codifies a transmem brane receptor (N3) of 2321 amino acids. Similar to other NOTCH receptors, NOTCH3 is synthesized as a complete protein, which suffers a proteolytic rupture (S1 rupture) by furine, generating two domains: the extra cellular Nterminal domain (N3ECD, 210 kDa) and an intracellular terminal (N3ICD)(Tikka et al., 2014). The main functions of these are during organogenesis, includ ing vascular genesis, stem cell renewal, cell proliferation, cell fate determination, and differentiation and apop tosis (Joutel et al., 2000; Prakash, Hansson, Betsholtz, Mitsiadis, & Lendahl, 2002).
This disease is hereditary in nature, with an autosomal dominant pattern, and is characterized by transient ischemic attacks (85%), migraine with aura (41%), and cognitive (50%) or psychiatric (20 − 41%) impairment, with a high prevalence of depression and apathy, as well as occasionally epilepsy (10%) (López & Vilanova, 2009). The progression of the disease leads to a major neurocognitive disorder of subcortical nature, neurological dysfunctions such as dysarthria, pseudobulbar palsy, and hemiparesis, and finally death, coming generally 1525 years after the first symptoms appear. Young adults of both sexes are affected (Di Donato et al., 2017; Wesołowski, Dziewulska, Koziarska, & IżyckaŚwieszewska, 2015).
With regard to its cognitive profile, CADASIL has a clinical evolution different to that of other forms of dementia, making it important to specify the cognitive function supporting the differential diagnosis. In this respect, (Dichgans, 2009) demonstrated that this pathol ogy creates a deficit in processing speed and executive functions, low verbal fluency, and concentration prob lems while episodic memory is preserved. In general, people with this disease present lapses both in immedi ate free recall and longterm memory, but recognition is preserved even in the case of elderly people and those in the moderate stage of the disease, suggesting that the encoding process is preserved even as the disease progresses ( Di Donato et al., 2017). Cognitive deficit reduces significantly with age, while impairments appear to instrumental and verbal activities, visual memory, reasoning and spatial skills (Buffon et al., 2006).
On another note, the results of neuropsychological tests of all the cognitive processes significantly corre late with the number of lacuna infarcts. A recent study demonstrated that the incidence of dementia in CADASIL is associated with the number of recurrent CVAs (Chabriat et al., 2016). However, few research projects have man aged to identify the relevant cognitive markers including those prior to the first CVA, although a slowdown in information processing and working memory have been observed (Brookes, Hollocks, Tan, Morris, & Markus, 2016).
It is estimated that there are around 500 families with CADASIL worldwide, in which more than 200 different mutations of NOTCH3 have been described, confirming high genetic heterogeneity. There is great variability in the phenotype even between members of the same family (Dziewulska, 2009; Rutten et al., 2016). Nonethe less, few studies have focused on the identification and differentiation of the cognitive profile of each of these mutations, although the discrimination between them is important in clinical analysis. In Colombia in 2010, a genotypephenotype was correlated in carriers of the R1031C and C455R mutations, with the conclusion that the participants with the R1031C mutation presented greater cognitive impairment and dementia, while with the C455R mutation, the age of onset was lower but cognitive deterioration was slower and less aggressive (Moreno et al., 2010).
Meanwhile, the R141C mutation described in this article has a prevalence of in the European popula tion, yet there have been few studies on people with this mutation. Only five related studied were found in the database consulted (PubMed). In Japan, while multiple families with CADASIL have been identified, to date only two subjects with the R141C mutation, unrelated to each other, have been described (Mizuno, Mizuta, & Tomimoto, 2016; Murakami et al., 2001; Önder, Kurtcu, Arsava, & Topcuoglu, 2017; Yadav, Bentley, Srivastava, Prasad, & Sharma, 2013).
In India, a family with CADASIL has emerged for the first time with 17 individuals spanning 3 compatible generations, of which 5 members have been confirmed as having this mutation (Yadav et al., 2013). Finally, in Önder et al. (2017) reported that the R141C mutation is uncommon, adding that only two research projects on the mutation had been carried out in the country: a case study in 2014 and a report on two individuals in 2017.
In Colombia no descriptions exist of the cognitive genotypephenotype of the R141C mutation. This study is the first to create such a description, and moreover, has a robust sample size, larger than those previously re ported in respect to other mutations linked to CADASIL. Considering the above, this study aims to establish the cognitive performance of a group of asymptomatic carriers belonging to families in the department of An tioquia with the R1031C and R141C mutations of the NOTCH3 gene. In addition, it aims to compare the neu ropsychological profile of people with the R1031C with that of a group of noncarriers. To achieve this objective, a key task is the implementation of neuropsychological batteries able to rapidly identify the cognitive dissoci ation of CADASIL and with enhanced discriminatory power through the use of evaluative measures that al low subtle changes to be detected even in asymptomaticm patients.
2. Method
2.1. Research Type and Design
An observation, crosssectional, and analytical study was performed, comparing the cognitive performance of a group of asymptomatic carriers of the R1031C mutation with that of a group of noncarrier subjects belonging to the same CADASIL families. Additionally, a group of carriers of the R141C mutation was compared to neuropsychological scales established in the normal pop ulation. Each participant was evaluated after providing informed, written consent. This project was approved by the Bioethics Committee of the University of Antioquia.
2.2. Participants
The participants in this study were asymptomatic carriers belonging to CADASIL families in Antioquia (Colombia). They had Functional Assessment Staging (FAST) and Global Deterioration Scale ratings between 1 and 2 and no history of CVA. The participants were contacted using information on the SISNE database of the Neuro sciences Group of the University of Antioquia Research Center (SIU). Participants were selected based on inclu sion criteria after neurological and neuropsychological evaluation. DNA extraction was carried out for all mu tations following the SIU procedure, with a test termed “PCR RFLP” using restriction enzyme digestion, and visualized in agarose gel.
The exclusion criteria were: subjects with a prior neurological illness other than CADASIL, a history of psychiatric illness or noncontrolled systemic disease, or illiteracy, which would prevent them from carrying out the neuropsychological evaluation.
The study was double blind; neither the participants nor the investigators were aware of the genetic status of the subjects, and therefore could not distinguish between the carrier and noncarrier groups. Simple random sam pling was performed using the SISNE platform, a process carried out by a systems engineer able to access and iden tify the carrier and noncarrier groups. Subsequently, subjects were divided into three groups according to their genotyping. The first group was composed of 39 asymp tomatic carriers with the R1031C mutation; the second of 8 asymptomatic carriers of the R141C mutation; and the third of 50 healthy individuals without the mutations affecting the CADASIL families.
2.3. Materials
The following procedures were performed with each par ticipant over two sessions:
Medical evaluation: Personal and family history, med ical examination with an emphasis on neurological symp toms, behavioral evaluation, and application of neuropsy chiatric scales (Cummings Neuropsychiatric Inventory [NPI], Clinical Dementia Rating [CDR] and application of dementia criteria from the Diagnostic and Statistical Manual of Mental Disorders [DSM IV]).
Neuropsychological evaluation: A neuropsychological evaluation protocol involving assessment of all cogni tive domains was applied, using guidelines validated in Colombia by the Neurosciences Group of the University of Antioquia. This included the following set of tests developed by the Consortium to Establish a Registry for Alzheimer’s Disease [CERADcol]: Verbal fluency test animals, Boston Naming Test (abbreviated format), MiniMental State Examination [MMSE], word list (re call and recognition of words on a list), constructional praxis (copying and recall), digit symbol, Trail Making test part A [TMT A], Raven test part A, verbal fluency test, phonological fluency test, Rey Osterrieth figure test, WAIS arithmetic test and Wisconsin Card Sorting test, modified version by the neurosciences group (Arboleda et al., 2010).
In addition, functionality and severity scales were applied including Functional Assessment Staging [FAST], the Global Deterioration Scale [GDS], Barthel (Mahoney & Barthel, 1965), Katz (Katz, 1963) and LawtonBrody (Lawton & Brody, 1969). To evaluate memory com plaints, the questionnaire was applied to the patient and family member, while for the emotional state evaluation, the abbreviated form Yesavage depression scale was used (Yesavage et al., 1982).
Following the recommendations of the studies car ried out in Colombia described above, it was decided to broaden the battery with respect to the evaluation of executive function, which includes the following neu ropsychological evaluation instruments:
2.4. Statistical Analysis
The SPSS 24 statistical package was used to analyze ob tained data. The statistics were used based on the nature of the variables. For quantitative variables, averages and standard deviations were obtained. The qualitative vari ables were analyzed in terms of frequency measurements and percentages. To establish the relationship with sociodemographic variables, a chi squared (x2) test was used, and to observe the differences in the performance of the cognitive tasks of each group, the MannWhitney nonparametric U test was used. A statistical significance level of 𝑝 < 0.05 was used.
Table 1 Additional Neuropsychological evaluation instruments.
| Neuropsychological Test | Cognitive Domain | Reference |
|---|---|---|
| Forward order number retention | Attention | Wechsler, 1987 |
| Free and cued selective reminding test [FCSRT] | Memory | Grober, Buschke, & Korey, 1987 |
| Trail Making Test B _ Time Ineco Frontal Screening [IFS], STROOP Backwards order number retention Letter and number sequencing | Executive Function | Reitan y Wolfson, 1985 Torralva, Roca, Gleichgerrcht, & López, 2009 Golden, 1976 Wechsler, 1987 Wechsler, 1987 |
| Matrices | Abstract Reasoning | Wechsler, 1987 |
| Geriatric Anxiety Scale | Functionality Scale | Pachana et al., 2007 |
3. Results
With respect to the demographic variables, it was found that the majority of the participants were women, with no significant differences being presented between asymptomatic carriers of the R1031C mutation (56.4%) and the noncarriers (66%). The results for education level showed that the carrier group had a median of 7 (medium education level), and the noncarrier group had a median of 5 (low education level), without significant differences being found between the groups. With regard to age, the asymptomatic carriers had a median age of 29 and the noncarriers 30 (see Table 2).
Comparison of the results of the neuropsychological tests of the asymptomatic carriers with mutation R1031C (𝑛 = 39) group and the noncarriers (𝑛 = 50), found statistically significant differences (𝑝 < 0.05) in constructional praxis cognitive processes of when copying the Rey Osterrierth figure (𝑝 = .010); for executive function in the INECO backwards digit span subtest (𝑝 = .023); INECO total (𝑝 = .024); INECO working memory scale (𝑝 = .011); and in the WAIS reverse order number retention subtest (𝑝 = .035). Similarly, statistically significant differences were found for abstract reasoning in the WAIS subtest matrices (𝑝 =, 029), with better cognitive performance observed in the carrier group with R1031C mutation, which is consistent with expectations for this population (see Table 3).
With regard to the demographic characteristics of the group of 8 asymptomatic carriers of the R141C mutation, 75% were women (in this regard there are no studies showing sex differences in the execution of the tests). The median education level was 7 (low level) and the median age was 37 (see Table 4).
Considering the neuropsychological results of the asymptomatic carriers with the R141C mutation, the cognitive performance of the group was described with respect to the Colombian scales validated for a normal adult population. The results for the MMSE general cognitive state evaluation showed an average of 28.13 points, while in the normal population the estimated average is 28.47 points with a standard deviation of 1.49. In tests evaluating the different cognitive domains, scores below those expected for the age range were observed for executive function (processing speed and working memory). In the Stroop word test (𝑛 = 3) the average score was 99 points compared to a standardized average of 33.1 points with a standard deviation of 11.2 for the normal population; and in the Stroop color test the average score was 58.50 points compared to an estimated average of 44.8 with a standard deviation of 12.6 points for the average population; while mental calculation assessed with the WAIS arithmetic subtest (𝑛 = 8) showed an average score of 5 points compared to the validated Colombian average of 7.7 points with a standard deviation of 1.8. Finally, the total INECO score (𝑛 = 3) results for the asymptomatic carriers of this mutation were below the expected level with an average of 24 points, while the baseline for the normal population is 26 points. While the scores for the TMTA time and digit symbol tests were not statistically significant, in the graph, longer time and lower performance can nonetheless be observed for the execution of these tests (see Graph 1).
Table 2 Demographic characteristics of the asymptomatic carriers of the R1031C mutation group and the noncarrier group .
| R1031C (𝑛 = 39) | Noncarriers (𝑛 = 50) | |||
| 𝑁 (%) | 𝑁 (%) | 𝑐2 𝑎 | 𝑝 Value | |
| Sex | ||||
| Male | 17(43.6) | 17(34) | 1.411 | 0.49 |
| Female | 22(56.4) | 33(66) | ||
| Med (IR) | Med (IR) | 𝑈 𝑏 | 𝑝 Value | |
| Education Level | 7(7) | 5(6) | 898 | 0.516 |
| Age | 29(14) | 30(12) | 955.5 | 0.87 |
Note: Med=Median; IR=Interquartile Range, the sign (+) indicates fo > fe. 𝑎 Pearson Chi squared; 𝑏 MannWhitney U ∗∗∗𝑝 < 0.001
4. Discussion
Returning to prior CADASIL studies in Colombia, in 2000,two families from Antioquia department carrying the R1031C and C455R mutations of the NOTCH3 gene were reported for the first time (Lopera et al., 2000). In 2007 another article was published that monitored the cognitive characteristics of these two mutations. This concluded that no differences were found between the subjects evaluated as they were young and asymptomatic, that cognitive decline over time was not expected and that a monitoring period of four years was not ade quate to determine significant evolution in cognitive al ternations. The article added that more sensitive tools were required for neuropsychological evaluation (Henao Arboleda, AguirreAcevedo, Pacheco, YamileBocanegra, & Lopera, 2007). Finally, in 2010 an analytical study was carried out in order to determine the genotypephenotype in this population, concluding that the R1031C mutation presented greater cognitive impairment and dementia, while carriers with the C455R mutation showed an earlier age for the onset of cognitive decline, although decline was slower and less aggressive (Moreno et al., 2010).
With respect to the mutations reported, it is impor tant to clarify that people with the C455R mutation are not included in the present study, as they did not fulfill the criteria. However, this study is the first to describe the R141C mutation, with no prior reports on this in Colombia and only a few case studies worldwide on sub jects from Europe, India, Turkey and Japan (Mizuno et al., 2016; Murakami et al., 2001; Önder et al., 2017). The cognitive profile of this mutation has not been described previously.
In the present study, cognitive analysis was per formed of 97 asymptomatic subjects with and without the NOTCH3 gene mutation. When 39 asymptomatic carriers of the R1031C mutation were compared to a group of 50 noncarriers from the same CADASIL fami lies (the participants in these two groups being mainly women) no statistically significant differences were found between carriers and noncarriers. Regarding education level, the two groups presented similar characteristics, which could be related to the cultural and economic cir cumstances of the subjects, who come from rural areas of the department of Antioquia. It is important to high light this information as the two groups presented similar demographic characteristics, facilitating analysis of the information.
The results demonstrated significant differences be tween the asymptomatic carrier group with the R1031C mutation and the noncarriers in cognitive tests evaluat ing constructional praxis and abstract reasoning, with inferior cognitive performance observed in the carrier group.
Considering the results of this mutation in construc tional praxis, while there were statistically significant differences in copying the Rey Osterrierth figure, this study does not suggest constructional apraxia. These results may instead be due to executive shortcomings, specifically with the construction type in the execution of the figure (planning and organization).
Meanwhile, the following INECO components showed significant results for executive function: backwards digit span, working memory scale, and total test score. This suggests that the R1031C carriers presented deficiencies in providing temporal information and in manipulating complex cognitive tasks (working memory). INECO was used because it has been shown to be a battery able to rapidly identify cognitive dissociations with increased discriminatory power in presymptomatic patients, and has demonstrated high sensitivity in 96.2% of cases, specificity in 91.5%, and predictive value (Torralva, Roca, Gleichgerrcht, Lopez, & Manes, 2010).
The study differs from most investigations, which refer to executive dysfunction occurring from the time of CVA recurrence, in that slight alterations were observed in the subjects evaluated. This is consistent with the work of Amberla and Brookes, which indicates that subjects with mutations in the NOTCH3 gene present alterations to short term memory, working memory and executive function even when in a preclinical state. From this it can be inferred that cognitive deterioration precedes the first ischemic event. Therefore, these cognitive characteristics could be the first symptoms of CADASIL (Amberla et al., 2004; Brookes et al., 2016).
Table 3 Results of the neuropsychological tests. Comparison between asymptomatic carriers of the R1031C mutation and noncarriers.
| R1031C (𝑛 = 39) | Controls (𝑛 = 50) | |||
| Function/test | Med (RI) | Med (RI) | 𝑈 𝑎 | 𝑝 value |
| Minimental | 29(3) | 29(2) | 860 | 0.317 |
| ATTENTION | ||||
| Digits and symbolscued | 48(29) | 42.5(21) | 228.5 | 0.791 |
| Forward order number retention | 7(2) | 7(2) | 188.5 | 0.228 |
| MEMORY | ||||
| Word list total (CERAD) | 18(6) | 18(5) | 895 | 0.507 |
| Total Intrusions | 0(1) | 0(1) | 919 | 0.602 |
| Word list evocation | 7(3) | 6(3) | 918 | 0.631 |
| Recognition | 10(1) | 10(1) | 961 | 0.885 |
| Praxial construction evocation (CERAD) | 8.50(3) | 8(4) | 880 | 0.428 |
| Osterrierth Figure evocation | 15.50(10) | 14.25(10) | 866.5 | 0.369 |
| FCSRT free 1 | 10(2) | 10(3) | 229.5 | 0.806 |
| FCSRT cued 1 | 15.50(1) | 16(1) | 212 0.463 | |
| FCSRT free 2 | 12(2) | 13(2) | 201 0.356 | |
| FCSRT cued 2 | 16(0) | 16(0) | 220.5 0.441 | |
| FCSRT free 3 | 15(1) | 14(2) | 177 0.133 | |
| FCSRT cued 3 | 16(0) | 16(0) | 221.5 0.430 | |
| FCSRT delayed free | 15(3) | 14(2) | 167.5 0.087 | |
| FCSRT delayed cued | 16(0) | 16(0) | 216.5 0.354 | |
| PRAXES | ||||
| Constructional Praxis (CERAD) | 10(2) | 10(2) | 793.5 0.117 | |
| Rey Figure | 25(7) | 29.25(8) | 662.5 ∗0.010 | |
| LANGUAGE | ||||
| Verbal Fluency | 16(7) | 16(5) | 927 0.69 | |
| Boston Naming (CERAD) | 12(2) | 13(2) | 966.5 0.943 | |
| EXECUTIVE FUNCTION | ||||
| Phonological Fluency ”F” | 10(8) | 8.50(6) | 816.5 0.326 | |
| Wisconsin success | 20(10) | 21(9) | 0.252 0.252 | |
| Wisconsin categories | 2(2) | 2(1) | 0.895 | 0.895 |
| Wisconsin perseverance | 19(9) | 17(9) | 0.299 | 0.299 |
| Trail Making Test A _ Time | 70(49) | 67(22) | 822.5 | 0.353 |
| Trail Making Test B _ Time | 143.5(95) | 120.5(105) | 158,5 | 0.94 |
| INECO Backwards digits | 4(2) | 5(2) | 144.5 | ∗ |
| INECO Total | 22(6) | 24(4) | 153 | ∗0.024 |
| INECO Working memory scale | 5(2) | 7(2) | 132.5 | ∗0.011 |
| STROOP Word | 90(31) | 82(28) | 225 | 0.729 |
| STROOP Color | 59(16) | 59(11) | 210 | 0.488 |
| STROOP ColorWord | 32(22) | 33(12) | 209 | 0.474 |
| Backwards order number retention | 4(2) | 5(2) | 151 | ∗0.035 |
| Letter and number sequence | 7(5) | 8(4) | 174 | 0,215 |
| ABSTRACT REASONING | ||||
| Raven | 9(1) | 9(3) | 929 | 0,986 |
| Matrices | 6(6) | 9.50(8) | 146 | ∗0, 029 |
| MENTAL CALCULATION | ||||
| Arithmetic | 9(2) | 7(5) | 815 | 0.389 |
| SCALES | ||||
| QF | 2(8) | 5(7) | 73.5 | 0,166 |
| QP | 7(13) | 11(16) | 788 | 0.221 |
| Yesavage | 1.50(4) | 3(6) | 827.5 | 0.294 |
| Geriatric Anxiety Scale | 1(8) | 2.50(8) | 202.5 | 0.377 |
Note: Med=Median ; IR=Interquartile Range, 𝑎 MannWhitney 𝑈 ∗𝑝 < 0.05; ∗∗𝑝 < 0.01; ∗∗∗𝑝 < 0.001
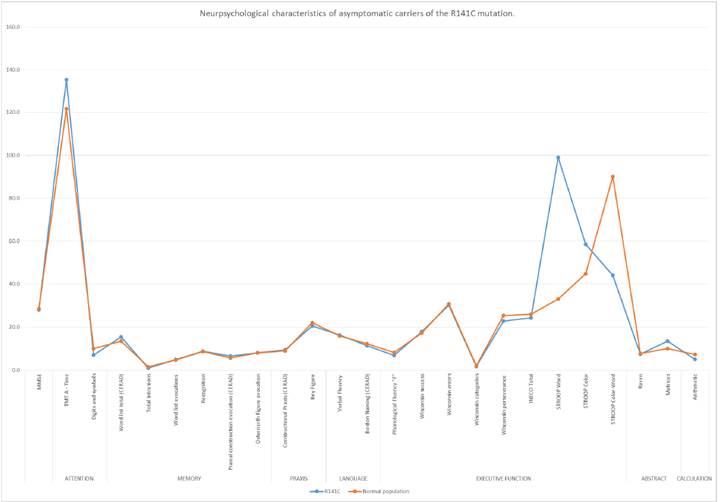
Figure 1 Neuropsychological characteristics of asymptomatic carriers of the R141C mutation. Colombian scale for normal population.
Table 4 Demographic characteristics of a group of 8 asymptomatic patients with the R141C mutation.
| R141C (𝑛 = 8) | |
| 𝑁 (%) | |
| Sex | |
| Male | 2(25) |
| Female | 6(75) |
Note: Med=Median ; IR=Interquartile Range, the sign (+) indicates fo > fe. 𝑎 Pearson Chi squared ∗∗∗𝑝 < 0.001
Similarly, statistically significant differences were found in tests measuring mental abstraction. Visuospatial func tions and reasoning have not been highly investigated in the CADASIL context and those studies that mention these skills refer to their preservation in the initial stages of the disease. Therefore, the current study differs from these and instead proposes deeper investigation into the cognitive domain of executive functions (Dichgans, 2009).
Another cognitive test implemented in this study was the FCSRT. This facilitates evaluation of memory encoding and recall, as well as identifying false recognition and intrusions. Although commonly used and demonstrated to be highly specific in the context of Alzheimer’s disease, a study by Epelbaum et al. (2011). showed that a third of those tested with CADASIL presented memory deterioration according to the FCSRT (free recall), this being the second most significant cause after alterations in executive function (attention deficiencies and working memory) (Epelbaum et al., 2011).
While in the current study the results for the FCSRT were not statistically significant, it is suggested that longitudinal studies be performed to demonstrate changes in the performance of this cognitive process. It would be expected that patients with NOTCH3 gene mutations would gain higher scores for cued recall than for free recall, suggesting that people with subcortical damage present greater difficulty in information retrieval, while those with cortical diseases present greater difficulties in encoding (Epelbaum et al., 2011; Russo et al., 2013).
Regarding the neuropsychological results obtained with the asymptomatic carrier group for the other evaluated mutation (R141C), the cognitive profile was de scribed using the scale used for the Colombian population in the neuropsychological evaluation protocol established by the Neurosciences Group of the University of Antioquia. This can be used for diagnosis, monitoring, orolder people, comparison with others with or without cognitive impairment (Arboleda et al., 2010).
In these tests, asymptomatic carriers of the R141C mutation presented an average of 28.13 points in the MMSE general cognitive state evaluation, with the average score for the normal population estimated as 28.47 with a standard deviation of 1.49 points. This indicates that the average global cognitive performance was within the expected range, which is expected in the case of healthy participants, as MMSE results in initial stages tend to be normal. For this reason, this study was too short to evaluate cognitive decline in the initial stages of this pathology (do Campo Vázquez, MoralesVidal, Randolph, Chadwick, & Biller, 2011).
In tests evaluating the different cognitive domains, scores below those expected were observed for executive function in the INECO, Stroop word and Stroop color tests. In the evaluation of mental calculation, scores below those expected were obtained in the WAIS arithmetic subtest. This suggests that in the sample group there were cases of slow information processing and executive and working memory dysfunction, something that is not well described in the literature and is still being explored as an initial symptom in asymptomatic subjects with NOTCH3 mutations (Amberla et al., 2004).
In addition, the results obtained in the Stroop tests (color and word) correspond to those reported by Vazquez et al., who indicate that subjects with CADASIL present speed reductions intimecontrolled tasks, a slowdown that also affects abstract reasoning (a difficulty found in the R1031C mutation) (do Campo Vázquez et al., 2011).
In conclusion, this study differs from previous investigations where findings suggested that asymptomatic subjects with NOTCH3 mutations, the presence of leukoencephalopathy and no CVA history do not predict alterations in cognitive performance (Torralva et al., 2010). In contrast, it supports studies that link frontal subcortical patterns with greater executive impairment, although as the disease advances and cognitive impairment worsens, the cerebral cortex also tends to be affected in CADASIL due to cortical micro infarcts or as a result of degeneration, which in studies of more advanced stages of the disease cause deterioration in memory (Di Donato et al., 2017).
The results of this study improve our understanding of the cognitive characteristics that differentiate a diagnostic group with the R1031C mutation to one with the R141C mutation, the neuropsychological characteristics of which are still being explored. Although both groups showed cognitive impairment, there were differences. Asymptomatic carriers of the R1031C mutation showed alteration in constructional praxis (planning and organization), working memory and mental abstraction compared to the control group. Meanwhile, asymptomatic carriers of the R141C mutation showed greater impairment of processing speed and operative memory, as well as executive dysfunction.
According to the information and studies described above, this study produces results that support indications that asymptomatic carriers of NOTCH3 mutations tend to present a greater deterioration in working memory and processing speed, suggesting that these changes are possible cognitive markers in CADASIL. For this reason, further investigations are proposed into clinical aspects that could predict the disease, as well as the use of controlled cognitive tests with eventrelated potentials measuring processing speed, reaction times and oddball paradigms, and the evaluation of Craik and Lockart processing level memory paradigms.
Finally, it is of paramount importance to mention that the ideal moment to begin the treatment of any neurodegenerative disease is before the appearance of clinical symptoms. Considering that CADASIL is considered to be the most common hereditary cause of recurrent ischemic strokes and is identified as a model of pure vascular dementia, it is ideal for study. Moreover, the families with CADASIL found in Colombia make














