1. Introduction
According to WHO, stress has been considered the epidemic of the xxi century (Fink, 2016). Between 1983 and 2020, the perception of stress increased among the general population due to an increment in stressors in the United States of America (American Psychological Association, 2020) and Mexico (Mexican Institute of Social Security, 2017). One of the first sources of stress was Covid-19 pandemic, which disrupted work, education, health care, economy, and relationships. In fact, cumulative stress has been associated with an increased probability of heart disease and hypertension (Albert et al., 2017), addictions (Roeckner et al., 2017), anxiety, depression (Trucco, 2002), acute stress disorder, post traumatic stress disorder (PTSD) (Sánchez-Castillo et al., 2014), Alzheimer’s disease, which is less reported, and in the case of stress occurring twice in life (pregnancy and puberty), and schizophrenia (Sarkar et al., 2019).
Stress is a highly personal phenomenon. It changes between subjects (Hammen, 2005), depending on their vulnerability, resilience (Couto-Pereira et al., 2019), and behavioral and cognitive resources to cope with adversity (Lehrer & Woolfolk, 2007). Chronic stress has been associated with the beginning, maintenance, and aggravation of the symptomatology of many psychiatric disorders such as anxiety (Martin et al., 2010) and major depression (Yin et al., 2016). Recently, there has been an increase in the number of psychological and behavioral research (Willner, 2015), investigations focused on the neurobiology of stress, its mechanisms, and consequences in animal models (Stanton et al., 2018), and improved technology for a better understanding that helps us to treat and prevent stress consequences (Fink, 2016).
2. Stress
It is hard to establish a precise moment when the history of stress concept begins. There are some predecessors like Beard "neurasthenia" or Cannon "homeostasis" (Hutmacher, 2021). Neither Beard nor Cannon properly defined the term stress, but their contributions allowed the construction of this concept. The concept of stress has changed among scientists since the first definition by Selye (1936), who defined it as "the non-specific response of the body to any demand" (1976, p. 137). However, researchers like Pacák (2001) show that different stressors cause specific responses measured by cFos activity in the brain, and these responses were also accompanied by neurotransmitter specifical changes. Selye’s definition could change to "body response to any demand". Over this definition, McEwen and Akil (2020) proposed stress was the body’s response to any real or perceived threat that enhances physiological and psychological activity to cope with the stressor. Also in the study of the stress response, the concept of allostasis and allostatic load were included, in which allostasis represents stability across change and allostatic load refers to the price the body pays for being forced to adapt to adverse psychosocial or physical situations. These concepts are important because they explain the systemic mechanisms that protect the system when it adapts and those that damage the system when it is overloaded (McEwen & Akil, 2020). Considering this, stress could be defined as a systemic response triggered by a stimulus potentially harmful to an organism (Migliaro et al., 2020).
Stress is highly associated with fear and anxiety. Craske et al. (2009) define fear as "an alarm response to imminent danger" (p. 1067) and anxiety as "the anticipatory response to a possible threat" (p. 1067). This conceptualization has remained and is approved by the American Psychiatric Association (2013). Even though those definitions represent a human state, they are useful in animal models either, for describing freezing and immobility behavior respectively (Fink, 2016).
The stress response begins when a stimulus is considered threatening and alters homeostasis (McCarty, 2016). If a stimulus is capable of initiating the stress response, it is considered a stressor (Sapolsky, 2019). However, although it seems that the stress response only begins in the face of high-intensity stressors, such as a predator attack, a natural disaster, or violence, the stress response also participates in the moments in which the body must deal with daily experiences such as social interaction, physical activity, and work (McEwen & Akil, 2020). When the brain senses a stimulus as a threat, it commands a response controlled by two large neuroendocrine systems, the hypothalamic-pituitary-adrenal (HPA) axis and the sympatho-adrenomedullary (SAM), which is part of the autonomic nervous system (ANS) (Fink, 2017). Understanding the neurobiological mechanisms that regulate the stress response is important to attend to the mental, behavioral, and somatic state of patients with stressrelated illnesses (Fink, 2016).
3. Neurobiology of Stress
The stress response involves a complex and evolutionarily conserved system where the central nervous system (CNS) mainly participates (Lovejoy & Michalec, 2017). To initiate the stress response, the system must first perceive a stressor. Stressors can be physical or psychological. Physical stressors must alter the physiological state of the system -bleeding, infection, pain, etc.-, while the psychological ones should be interpreted as aversive or challenging -dangerous environments, work demands, social disapproval, harassment, etc.- (Godoy et al., 2018). Although the nature of the stressor is different, it triggers a neuroendocrine response mediated by the activation of SAM and the HPA (Radley et al., 2017).
The SAM axis is the first to be activated when the stress response begins (Sharpley, 2009). The activation initiates in the brain stem, specifically the nucleus of the solitary tract (NTS) (see Figure 1), as well as the circumventricular organs because they process information related to blood pressure, respiration, somatic and visceral pain, and inflammation (Herman et al., 2003). Deregulation of these processes by some stressor signals the NTS and consequently activates the Locus Coeruleus (LC) that modulates the sympathetic response of the preganglionic sympathetic nuclei of the intermediolateral column of the spinal cord (Kandel et al., 2013). This allows the release of adrenaline and noradrenaline (NA), which increases the function of organs such as the heart, lungs, viscera, bladder, and adrenal glands, elevating blood pressure and respiratory rate, decreasing intestinal motility, promoting fluid retention, modulating glucocorticoid release, and releasing epinephrine and norepinephrine peripherally (Smeets, 2010). To regulate the sympathetic response, the NTS also activates the parasympathetic branch by activating the postganglionic nuclei of the spinal cord, and through the release of acetylcholine (Ach), they decrease sympathetic activity and return to a state of rest to start again (Ulrich-Lai & Herman, 2009).
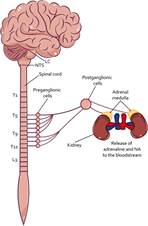
Note. The stress response begins with the activity of the SAM axis. First, the NTS signals to the LC the physiological dysregulations in the organism, which leads to the activation of the sympathetic branch at the level of the spinal cord. Then, through the preganglionic cells signals to the postganglionic cells, releasing NA, which promotes activation of the organs that innervate, for example, the adrenal medulla, promoting the release of adrenaline and NA peripherally. LC Locus coeruleus; NTS Nuclei of the solitary tract; NA Noradrenaline.
Figure 1 SAM Axis Activity
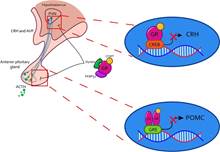
In addition to the activity of the SAM axis, the HPA axis is also activated during the response to physical stressors (see Figure 2). This occurs by the peripheral release of adrenaline and NA, which, through stimulation of the vagus nerve, promote the activity of the paraventricular nucleus of the hypothalamus (PVN) (Raison et al., 2006). This allows the synthesis and release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP), which travel through the pituitary portal blood to the anterior pituitary (Fink, 2017), promoting the synthesis and release of adrenocorticotropic hormone (ACTH) by binding to the CRH1 and CRH2 receptors (Faught et al., 2016). Once ACTH is released into the bloodstream, it travels until it reaches the adrenal gland, where, through the proopiomelanocortin (POMC) receptors, promotes the synthesis and release of glucocorticoids such as cortisol -main glucocorticoid in humans, dogs, hamsters, and fish- or corticosterone - main glucocorticoid in rats, mice, birds, and reptiles- (Faught et al., 2016; Doczy et al., 2009).
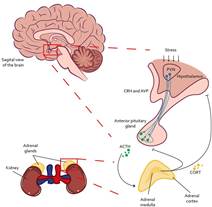
Note. Once a stressor is interpreted, the HPA axis initiates its response through the PVN at the level of the hypothalamus, where it secretes CRH and AVP to the median eminence. Once it reaches the anterior pituitary, it synthesizes and releases ACTH to the bloodstream. When ACTH reaches the adrenal cortex, it signals for CORT to be produced and released. PVN Hypothalamic Paraventricular Nucleus; CRH Corticotropin Releasing Hormone; AVP Arginine Vasopressin; ACTH Adrenocorticropic Hormone; CORT Glucocorticoids (cortisol or corticosterone).
Figure 2 HPA Axis Activity
In addition to signaling through the NTS for physical stressors, other kinds of stressors, such as psychological ones, initiate the stress response if the stressor is compared with past experiences and is interpreted as aversive or challenging (Skoluda et al., 2015). This activity is compared at the hippocampal and caudate nucleus level for the modulation of memory information and subsequently signals towards the basolateral amygdala (BLA), which transmits this information through the lateral amygdala (LA) and to the central nucleus of the amygdala (CeA) (Pruessner et al., 2010). Also, the basal amygdala (BA), which is part of the BLA complex, signals towards CeA or the medial amygdala (MeA) (Roozendaal et al., 2009). CeA and MeA control the autonomic and PVN response. However, CeA is the main source of efferent connections with the autonomic and endocrine systems to promote the physiological and behavioral response to the stressor (Vyas et al., 2003). Also, this response can be sensitized if exposure to stress is repeated (Radley et al., 2017). It should be noted that connections of CeA to PVN to control the endocrine response are scarce, the activation of the HPA axis is carried out through the inhibition of the bed nucleus of the stria terminalis (BNST), since BNST promotes the inhibition of PVN by activation of perihypothalamic GABAergic interneurons (Vyas et al., 2003).
Currently, the study of the neurobiology of stress is very complex, since it involves various structures of the CNS as well as the adrenal glands in the periphery (see Figure 3). The connection and proper functioning of these structures allow the adaptation of physiological and behavioral responses to increase the possibility of survival of the organism in the face of stressors (SánchezCastillo et al., 2014).
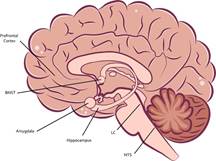
Note. The stress response begins with the SAM axis response, which involves signaling from the NTS to the LC, which increases sympathetic activity in the spinal cord. On the other hand, projections of the amygdala towards the BNST increase the activity of the HPA axis. initially in PVN, which signals to the anterior pituitary and later to the adrenal glands, promoting the release of glucocorticoids, epinephrine, and NA to deal with a stressful condition. In addition, structures such as the hippocampus and the prefrontal cortex modulate the activity of the HPA axis downward. BNST Bed nuclei of stria terminalis; LC Locus coeruleus; NTS Nuclei of the solitary tract.
Figure 3 Scheme of Structures at the CNS Level that Participate in the Stress Response
4. Glucocorticoids
The activation of the HPA axis is the release of glucocorticoids (cortisol or corticosterone) through the adrenal cortex, which cause a wide variety of transcriptional and non-transcriptional effects in the body (Doczy et al., 2009). These effects are related to metabolism (Jaszczyk & Juszczak, 2021), sodium and water balance (de Kloet, 2013), immune response (Raison et al., 2006), increased growth factors (Juszczak & Stankiewicz, 2018), cardiovascular function (Grippo et al., 2005), reproduction (Sánchez Castillo et al., 2014), mood, and cognitive functions (Timmermans et al., 2019), being its release and signaling of vital importance for the proper functioning of organisms (Becker, 2013).
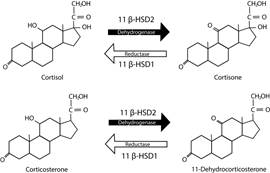
Their synthesis begins with cholesterol and ends with the production of mineralocorticoids, glucocorticoids, and androgens (Murison, 2016). Aldosterone is the main mineralocorticoid, and its function is related to the conservation of sodium and water. Cortisol is the main glucocorticoid in humans, dogs, hamsters, and fish, as well as corticosterone is the main glucocorticoid in rats, mice, birds, and reptiles, and its function is associated with gluconeogenesis (Faught et al., 2016; Doczy et al., 2009), as it can be seen in Figure 4, and dehydro-epiadrosterone (DHEA), which is the precursor of testosterone and estrogens (Timmermans et al., 2019).
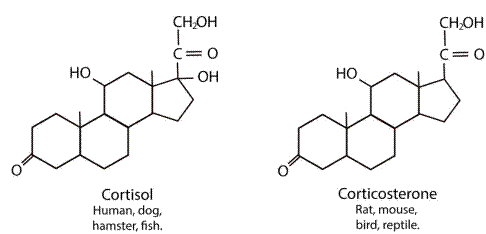
Figure 4 Structure Diagram of the Main Endogenous Glucocorticoids (Cortisol and Corticosterone) in Different Species
Its release is mainly mediated by the hypothalamus and to a lesser extent by the sympathetic branch of the autonomic nervous system (ANS), the thymus, the vasculature, and the epithelial barriers (Timmermans et al., 2019). Under normal conditions, glucocorticoids are released into the bloodstream in a circadian (rhythmicity in periods of approximately 24 hours) and ultradian (rhythmicity in periods of 20 hours or less), having their peak concentration during the active phase of the organism and decreasing towards the inactive phase (Dean & Keshavan, 2017). Around 10-20 mg are secreted daily unless the cycle is altered by stimulation of its release due to stress (Becker, 2013).
Glucocorticoids are highly lipophilic and when they are released into the bloodstream, they can pass through the cell membrane (Doczy et al., 2009). However, because they are highly lipophilic, to travel in the bloodstream, they are also coupled to proteins such as albumin (low affinity) and with high affinity to the globulin coupling protein (CBG) or transcortin, so that the glucocorticoids coupled to CBG are not free to cross the membranes of all cells (Schaaf & Meijer, 2017). The proportion of free glucocorticoids to bind to their receptors will depend on the amount of GBG available (Spencer & Deak, 2017).
On the other hand, glucocorticoids have a short halflife (<15 min in rodents and humans), due to their rapid metabolism by the enzyme 11β Hydroxysteroid dehydrogenase 2 (11β-HSD2) that converts corticosterone or cortisol into Deohydro corticosterone or cortisone, two hormones that have a very low affinity for glucocorticoid receptors and mineralocorticoid receptors, while 11β-HSD1 converts metabolites to active steroids (Hammond, 2016), as shown in Figure 5. The expression of 11β-HSD2 is higher in the kidneys and in the placenta, in pregnant women, protecting the fetus from the passage of glucocorticoids. Another important aspect is that the expression of 11β-HSD1 is higher in the hippocampus. Therefore, this is possibly one of the reasons why this structure is highly affected by stress (Spencer & Deak, 2017).
5. Glucocorticoid and Mineralocorticoid Receptors
Once glucocorticoids are uncoupled from CBG or albumin, they can either bind to membrane receptors and promote non-genomic effects or cross the cell membrane to promote their genomic effects (Dejean & Richard, 2013). To generate these changes, they require binding to two types of receptors (Hammond, 2016): glucocorticoid receptors (GR), where they bind with an affinity of 5-10 nM, and mineralocorticoid receptors (MR), with which they have an affinity of 0.5-1 nM (Doczy et al., 2009), that is, a higher concentration of glucocorticoids present is required to occupy the GRs, while in small concentrations, binding to MRs is possible (Schaaf & Meijer, 2017).
Under physiological conditions, during the inactive phase oforganisms (at night for humans and during the day for rodents), the receptors most occupied by the release of glucocorticoids are the MR (Reul & De Kloet, 1986). While the glucocorticoid release peak occurs during the active phase, the GR’s most occupied (Becker, 2013). Both GR and MR exert primary biological actions through gene transcription or non-genomic mechanisms (O’Connor et al., 2000). Both are members of the steroid hormone receptor superfamily, possessing DNA-binding domains that are available after ligand binding (Doczy et al., 2009).
Receptors found in the cytosol as monomers are coupled to available glucocorticoids, associated with a complex of chaperones that include heat shock proteins (HSP) and immunophilipins such as FKBP51 and FKBP52 (Faught et al., 2016), as shown in Figure 6. When this is achieved, a translocation signal to the nucleus is activated through the activity of HSP (Pratt et al., 2004). Once in the nucleus, the GR and MR have an affinity to DNA for immunophilipins (Kirschke et al., 2014). Mediating cellular activity, coupling directly to DNA response elements such as homodimers (GR-GR) or heterodimers (GR-MR) (1), or interacting with other transcription factor complexes (2) (Timmermans et al., 2019).
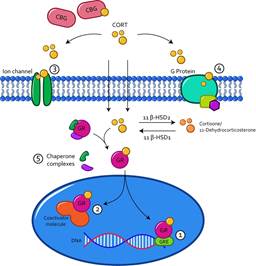
Note. Glucocorticoids that uncouple from CBG or albumin can cross the cell membrane and couple within the cytosol to their GR receptors and translocate to the cell nucleus. In there they can have direct effects on gene expression by coupling to GREs in DNA (1) or indirectly through coupling to a coactivator molecule (2). Among the genomic effects is the modulation of cell signaling, through the activation of ion channels (3), or G protein-coupled receptors (4), as well as the dissociation of the chaperone complexes from the GR receptors. (5). CBG globulin coupling protein; CORT cortisol or corticosterone; β HSD, Beta hydroxysteroid dehydrogenase; GR Glucocorticoid receptor; GRE Glucocorticoid response element.
Figure 6 Genomic and Non-genomic Action of Glucocorticoids
GR and MR have a differential distribution in the body (Dejean & Richard, 2013). While GR is widely distributed in most cells of the body, at the CNS level they are expressed in large quantities in the hippocampus, amygdala, PVN, and prefrontal cortex (PFC), while MR has a more restricted expression. They are mainly expressed in the limbic system, with high expression in the hippocampus, and moderate expression in the amygdala and PFC (Koning et al., 2019), as well as in the kidneys (Spencer & Deak, 2017). GR and MR control various processes such as neuronal differentiation (Fitzsimons et al., 2013), neuron excitability (Joëls et al., 2017), as well mood and cognitive processes such as memory (de Kloet et al., 2005). However, its effects are not only limited to the brain, at the peripheral level, but they can also modulate the immune response, where MR receptors promote the pro-inflammatory response and GR, the anti-inflammatory response (Chantong et al., 2012).
6. Terminating the Physiological Stress Response
The SAM axis is the first to decrease its activity, after a stressor exposure (see Figure 7). This regulation occurs by the activation of the NTS that stimulates the parasympathetic branch and allows the release of Ach (Ulrich-Lai & Herman, 2009), which decreases the activity of the heart, lungs, viscera, bladder, and adrenal glands, decreasing blood pressure, respiratory rate, fluid retention, the release of glucocorticoids, restoring intestinal activity, and reducing the release of adrenaline and noradrenaline (Smeets, 2010).
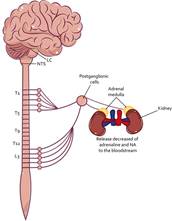
Note. Once the activity of the SAM axis begins, the NTS activates the parasympathetic branch of the autonomic nervous system. That, through the cervical and sacral region, signals the preganglionic cells that communicate to the postganglionic cells releasing Ach, which promotes the inhibition of the organs that innervate, for example, the adrenal medulla, decreasing the release of adrenaline and NA peripherally. LC Locus coereleus; NTS Nuclei of the solitary tract: NA Noradrenaline.
Figure 7 Regulation of the SAM Axis
On the other hand, the activity of the HPA axis, by a stressor, decreases due to the activity of the limbic system and glucocorticoid activity (Pruessner etal., 2010). Glucocorticoids released into the bloodstream, when the stress response is triggered, generate a phenomenon known as negative feedback. This phenomenon occurs when a system regulates itself, in this case, the release of glucocorticoids decreases the activity of the HPA axis at different levels, reducing the release of corticotropins through coupling to the GR and MR (Doczy et al., 2009). These effects are generated by signaling at the level of the anterior pituitary gland and hypothalamus due to that these structures present large amounts of GR (Mizoguchi et al., 2001).
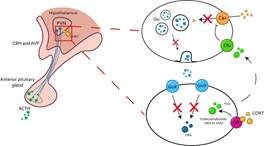
Negative feedback regulation occupies genomic and non-genomic mechanisms (Gjerstad et al., 2018) to decrease the HPA axis response (Osterlund et al., 2016). The genomic mechanisms occur through the repression of the transcription of the CRH and POMC genes, which decreases the translation into proteins of CRH and ACTH (Myers et al., 2012), as shown in Figure 8. However, regulation of HPA axis activity can also be regulated by nongenomic mechanisms by promoting endocannabinoid release from CRH-releasing neurons and inhibiting glutamate release from excitatory presynaptic neurons (Timmermans et al., 2019), as it can be seen in Figure 9. Regulation of the stress response by non-genomic GR signaling lasts approximately seconds or minutes (Groeneweg et al., 2012), while genomic mechanisms of GR take approximately 45 to 60 min (Malham et al., 2002). It is a slow regulation; however, this delay protects the system from constantly responding to stressful events (Willner, 2017).
Note. This process can occur in the PVN or in the pituitary gland. In both, CORT translocates to the nucleus coupled to a GR and inhibit the transcription of CRH and ACTH genes, decreasing the signal to continue the stress response. PVN Paraventricular nucleus of the hypothalamus; CRH Corticotropin releasing hormone; AVP Arginine vasopressin; ACTH Adrenocorticotropic hormone; GR Glucocorticoid receptor; CREB cAMP response element-binding protein; GRE Glucocorticoid response element; POMC Proopiomelanocortins.
Figure 8 Regulation of the stress response by genomic activity of the GR
Note. CORT can regulate endocannabinoid signaling, from postsynaptic cell to presynaptic cell and reduces calcium flux decreasing Glu release from excitatory presynaptic neurons, therefore CRH signaling is downregulated. PVN Paraventricular nucleus of the hypothalamus; CRH Corticotropin releasing hormone; AVP Arginine vasopressin; ACTH Adrenocorticotropic hormone; GR Glucocorticoid receptor; Ca+ Calcium; Glu glutamate; GluR Glutamate receptor.
Figure 9 Regulation of the stress response by the nongenomic activity of the GR
Also, limbic system structures related to emotional processing participate in the regulation of the HPA axis (Pruessner et al., 2010). Neurons of the PVN of the hypothalamus receive GABAergic inputs from BNST and excitatory inputs from various nuclei of the brain stem, including neurons of the NTS (Radley et al., 2017; Ulrich-Lai & Herman, 2009). GABAergic circuits of the BNST receive glutamatergic inputs from the hippocampus (in the region of the ventral subiculum) and the PFC (i.e., the medial PFC, the anterior cingulum, and the prelimbic PFC), resulting in inhibition of the activity of the PVN and completion of the stress response (Vyas et al., 2003), as shown in Figure 10. However, BNST receives GABAergic inputs from structures such as the amygdala (in its medial and central region), which decreases the inhibition of the PVN (by the BNST), and stress response can be initiated or maintained for a longer time. On the other hand, the PFC (in its infralimbic region) signals through its glutamatergic connections to the PVN of the hypothalamus, which would also increase or maintain the stress response (Doczy et al., 2009).
7. Acute and Chronic Stress
One of the main controversies in stress research is the temporality of exposure to one or more stressors. Short exposures to stress have been understood as acute stress, while prolonged exposures are called chronic stress (Stoney, 2017). Initially, the acute stress response was defined as exposure to a stressor that does not exceed a short time from minutes to hours (Dickerson & Kemeny, 2004), while the chronic stress response was defined as exposure to a stressor that lasts for days, weeks, or even years (Joëls & Baram, 2009).
Different situations have the potential to become stressors and we deal with them multiple times a day (e.g., work stressors and social stressors). The first exposure to them would provoke an acute stress response that would allow us to deal with the situation in a potentially successful way. If the stressful situation were repeated several times (the same day, during the week, during the month, or the year), it would at some point become a chronic stressor (Rohleder, 2019). However, one question arises when we consider this problem: When does acute stress become chronic?
Authors such as Katz et al. (1981) conducted experiments testing stress batteries in rats to study the possible relationship between stress exposure and altered behavior. Their results indicated that exposure to stress for 21 days had greater effects on behavior than exposure to stress for 1 hr. As their stress batteries reached 21 days of exposure, and when replicating their studies many other authors found similar results (Beck & Luine, 1999; Guo et al., 2009; Zlatkovi´c et al., 2014), the chronic stress response was believed to only achievable up to 21 days. However, over the years, it was detected that, in addition to the temporality, the intensity of the stress, the predictability, as well as its nature were important factors to achieve the altered behavioral phenotype (Willner, 2017). Currently, several studies called their protocols chronic when the exposure lasts 10 days (Valencia-Flores, 2018), 14 days (Tseilikman et al., 2020), 21 days (Roohi-Azizi et al., 2018), and 56 days (Chen et al., 2016).
Further, authors like Rohleder (2019) define acute stress as stressors of limited time, brief, low intensity, and a very similar environment (Rohleder, 2019), while chronic stress is defined as sequences of stressful events, in which an initial stressor leads to a series of repeated stressful events. Chronic stressors are considered highintensity and have a negative effect in the short or long term (Rohleder, 2019). Furthermore, authors like Nursey and Phelps (2016) added a category that includes traumatic stressors: these are acute high-intensity stressors that directly threaten life, which occurred in the past, and do not occur again in the present, but have long-term consequences in the psychological health of the individual.
In summary, acute stress occurs in a short time (less than 24 hours) but has no short or long-term consequences, and chronic stress is made up of acute events, but sequenced, which continue for a certain time (more than 24 hours) and produce short or long-term consequences. Finally, traumatic stressors are events that directly threaten the life of the body and have long-term health consequences.
8. Cognition, Brain Plasticity, and Stress
The stress response not only involves the source of stress, the mobilization of energy resources, and the physiological regulation of the organism, but it also has an important cognitive component for its triggering, as well as its regulation (Lehrer & Woolfolk, 2007). This cognitive control is used in therapeutics to reduce the physiological symptoms of people with stress-related disorders (Dwyer et al., 2016). From a psychological perspective, authors like Lazarus and Folkman (1984) mention that the stress response involves the relationship between the subject and its environment, where the demands of the environment are perceived as uncontrollable for the individual. Although this theory of cognitive control of the stress response was raised in the 1980s, it is still used today as a central tool in therapeutic research (Berenguera et al., 2020). Classification of the event as a potential stressor falls on the evaluation, as a cognitive process that allows evaluating an event and giving it significance for the individual. Thus, psychological stressors to trigger the stress response must be interpreted as threatening (Calvo & Gutiérrez-García, 2010).
The cognitive evaluation of the stressor can be of two types: primary and secondary (Carver & Connor-Smith, 2010). In turn, as Calvo and Gutiérrez-García (2010) reported, the primary evaluation can be of three types: (1) irrelevant, the situation is evaluated as neutral for the individual and does not have a stressful consequence; (2) benign, the situation is evaluated as positive and is not stressful; and (3) stressful, the situation is assessed as relevant to the organism (Calvo & Gutiérrez-García, 2010). If seemed stressful, the secondary assessment begins with the resources, capabilities, and options to deal with the demand considered. In this evaluation, the stressor can be considered (a) threatening, an event that may have negative consequences is perceived; (b) harmful, the perception that something negative has occurred; (c) loss, access to something has been lost which is positive; and (d) challenging, is evaluated as an opportunity to make a profit (Calvo & Gutiérrez-García, 2010).
The evaluation of the stimulus is not always directed, as in many cases this evaluation works automatically (Carver & Connor-Smith, 2010). In addition, the perception of a stressful event is modulated by unpredictability and uncontrollability. These two perceptions are triggered by any situation. However, during the stress response, the inability to predict what, when, where, how, and with what intensity or the perception of not being able to deal with the stressful situation increases the intensity of the stress response, its duration, as well as the consequences (Calvo & Gutiérrez-García, 2010).
On the other hand, the stress response, depending on whether it is acute or chronic, modulates cognitive functions such as attention, memory, learning, and executive functions (Kim & Diamond, 2002). Specifically, during the acute stress response, attentional processes improve and focus on the stressor, and emotional memory processing for negative events, as well as learning for negative emotional events, improve (Sánchez-Castillo et al., 2014). Cognitive flexibility decreases and increases rigid responses such as habits and decision-making oriented towards riskier choices (Calvo & Gutiérrez-García, 2010; Marin et al., 2019).
During the chronic stress response, the effects on cognitive functions can be modulated up or down (Orsetti et al., 2007; Wolf, 2017). Attention can decrease or stay increased, memory and learning are affected downwards, cognitive flexibility decreases, and decision-making is also altered towards higher risk options, in addition, the reinforcing value of stimuli decreases (Calvo & Gutiérrez García, 2010; Marin et al., 2019).
These cognitive changes, the product of exposure to stress, are associated with structural and functional changes in regions of the limbic system such as the hippocampus, prefrontal cortex (PFC), and amygdala (Pêgo et al., 2009; Qiao et al., 2016). In the hippocampus, exposure to chronic stress produces neuronal atrophy and reduces neurogenesis. These alterations have been associated with an increase in the presence of glucocorticoids, which reduces the number, dendritic length, and quantity of new neurons. These changes have been associated with increased glutamatergic signaling of N-methyl aspartate (NMDA) receptors (McEwen et al., 2016).
In contrast to atrophy and reduced hippocampal neurogenesis, exposure to chronic stress promotes hypertrophy of amygdala neurons (Vyas et al., 2002). Chronic stress increases the dendritic arborization of pyramidal neurons in the BLA, as well as glucocorticoid and CRH levels in this region. This effect is associated with an increase in glutamatergic signaling by NMDA receptors and a decrease in GABAergic signaling (Radley et al., 2017). Hippocampal atrophy, together with the hypertrophy of the amygdala, promotes a state of hyperactivity of the HPA axis, which promotes cognitive alterations in attention, memory, and anxiety-like and depression-like behavior (Willner, 2017).
The plasticity of neurons in the PFC, mainly in the regions of the anterior cingulate, prelimbic cortex, and infralimbic cortex, are altered by exposure to chronic stress mostly by reduction of the length, density, and arborization of dendritic spines of pyramidal neurons in the apical region. In addition, these changes have been associated with increased levels of glucocorticoids (McEwen & Morrison, 2013).
9. Stress and Ageing
Throughout our lives, we experience situations or environmental demands in which we adjust our response to this dynamic environment, so the stress response is of utmost importance. However, we must consider that the stress response is different during the various stages of our life, from fetal development to old age. This is because the organism, during the first stages of life, begins its development, and as time passes it goes maturing and adjusting the responses to maintain heterostasis (e.g., personal communication). Furthermore, when we become older (ageing), we suffer a gradual functional decline process (McHugh & Gil, 2018) that affects every way in which we respond to stress. Although its biological causes remain largely unknown, studies in the past few decades have identified common cellular and molecular traits associated with aging (López-Otín et al., 2013). For example, senescence refers to a cellular response that limits the proliferation of aged or damaged cells. Although senescence plays physiological roles in normal development, it has also been implicated as a major cause of age-related changes (McHugh & Gil, 2018).
The first stage of development is the prenatal stage. In humans, this stage lasts about 9 months, and in rodents, it lasts about 3 weeks (McCormick & Hodges, 2017). During this stage, the brain continues to develop, and the main processes that occur during this stage are neuronal differentiation and myelination (Conrad, 2011). Once the third week of gestation in rats ends, and by the second trimester in humans, the HPA axis becomes functional (Walker & McCormick, 2009). Although their regulation is not fully mature, the neurons of the PVN begin to respond to physical stress and glucocorticoids start to secrete from the adrenal gland (McCormick & Hodges, 2017).
During this stage, the placenta plays a very important role in regulating the mother’s stress and its effects on the fetus (Krontira et al., 2020). It has a large amount of NE and adrenaline transporters, as well as enzymes such as 11β-HSD2, to break down glucocorticoids to their inactive metabolites and reduce the impact of stress on the developing body (Piquer et al., 2017). However, it also has high amounts of the enzyme 11β-HSD1 that converts metabolites to active glucocorticoids, since its presence is vital for the development of the fetus to the neonate (McCormick & Hodges, 2017).
The second stage of development is the neonatal stage. In humans, it lasts approximately up to 4-5 years of age, while in rodents it lasts approximately 21-25 days (Rice & Barone, 2000). This stage is characterized by being a relatively long period of great parental care because humans, such as rodents, are altricial organisms, that is, they are born helpless, without the ability to support themselves (Conrad, 2011). During this period, neurogenesis decreases considerably, while gliogenesis remains in the process (Rice & Barone, 2000). In addition, the maturation of the cortex, as well as the cerebellum and hippocampus, continues for about 3 weeks later in rodents and the first 4 years in humans (McCormick & Hodges, 2017).
In the neonatal stage, the regulation of the stress response is highly dependent on the mother, the release of glucocorticoids begins to adjust at a rate, around the first two weeks (Spiga et al., 2014), MR and GR reach their peak of expression around 2 and three weeks, respectively. In addition, the response of the HPA axis shows resistance to activation during the first two weeks and is known as the hyporesponsive period of stress (McCormick & Hodges, 2017).
The third stage of development is adolescence. This period ranges from 11 to 16-17 years in humans and from 21 to 60 days approximately in rodents (Romeo, 2014). During adolescence, the puberty period becomes present; it begins earlier in females (the vaginal canal opens) and in males (the balanopreputial separation). The regulation by gonadal steroids (estradiol or testosterone) begins to promote differences in regulation of the HPA axis and the brain (Spear, 2000). During this stage, neurogenesis decreases in the hippocampus, but remains even higher compared to adulthood. Cell proliferation is noticeable in the amygdala, locus coeruleus, nucleus accumbens, and prefrontal cortex (Andersen & Teicher, 2008). Furthermore, it increases synaptic pruning and cell reorganization (Glover & Clinton, 2016).
During this period, sexual differences appear due to the release of gonadal steroids, testosterone modulates the response of the HPA axis downward, while estradiol increases its activity (Juraska et al., 2013). However, the development of PVN, the expression of GR and MR, and the basal glucocorticoid levels are very similar to those of adults (McCormick & Hodges, 2017). The difference is that, during adolescence, the release of glucocorticoids is more prolonged, as well as the effectiveness of CRH, which is greater compared to adults (McCormick & Mathews, 2010).
The fourth stage of development is adulthood. This period ranges in humans from 17-18 years to approximately 65 years and in rodents from 60 days to approximately 24 months (Conrad, 2011). In adults, neurogenesis declines to its lowest peak, without completely disappearing. The cortex and hippocampus, as well as other structures, have reached their peak of maturation. Hormone levels stabilize and risky behaviors as well as gambling behavior decrease (Chaby et al., 2017).
During this stage, the release of glucocorticoids in the face of a stressor decreases, since the better negative feedback of the HPA axis is achieved, and the circadian rhythmicity is consolidated, having release peaks at the beginning of the activity phase and decreasing towards the inactivity phase of the organism (Steptoe & Serwinski, 2016). In turn, during adulthood, the consequences of exposure to stress do not have long-term effects as in previous periods; adults show recovery from stress in approximately 10 days (Conrad, 2011).
The last period of an organismťs life is aging. This stage includes humans from 65 years of age and rodents from approximately 2 years of age (McCormick & Hodges, 2017). Aging is associated with the selective loss of mental abilities and a high risk for the development of neurodegenerative disorders. However, aging is experienced in different ways depending on the care taken during the previous stages (Conrad, 2011).
During aging, the HPA axis response is altered, negative feedback is diminished, the response is exacerbated by a stressful condition, and the circadian regulation of glucocorticoid release loses its rhythmicity (Garrido et al., 2012). In addition, exposure to stress during this period potentiates the adverse effects of stress, decreases cell proliferation, dendritic density, and synaptic contacts, and even some reports indicate that the possibility of death increases if stress becomes chronic during old age (Van Camp et al., 2018).
10. Psychiatric Illness and Stress
The relationship between stress and psychiatric disorders is widely reported (McEwen & Akil, 2020; Neumann et al., 2011; Nursey & Phelps, 2016). Stress plays a very important role in the onset, maintenance, and worsening of some psychiatric disorders (Chaby et al., 2017). Dysregulation of the stress system and allostatic load are implicated in many psychiatric disorders (McEwen & Akil, 2020). In fact, in the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM5, 2013), symptoms associated with stress appear in disorders such as schizophrenia (Corcoran et al., 2002), autism (Derguy et al., 2016), posttraumatic stress disorder (Porter et al., 2016), major depression (Belzung et al., 2015), in substance abuse disorders (Favoretto et al., 2020), and anxiety disorders (Riboni & Belzung, 2017).
Although exposure to stress has been linked to different mental disorders, some of the most studied are anxiety and depression disorders (Pêgo et al., 2009). Studies point to stress as a risk factor that increases allostatic load during life for the development of a psychiatric disorder (Fink, 2016). Anxiety disorders are characterized by excessive fear and avoidance in response to specific objects, situations, or sensations and the absence of true danger (Craske et al., 2017). The relationship between stress and anxiety disorders lies in the similarity of affectations in the neural circuits that are responsible for these responses, such as the limbic system and the hyperactivated sympathetic response (Shin & Liberzon, 2010). One of the most important biomarkers in anxiety disorders is cortisol levels (Roozendaal et al., 2009). In disorders such as post-traumatic stress disorder (PTSD) in generalized anxiety disorder, glucocorticoid levels are chronically decreased (Daskalakis et al., 2016).
One of the vulnerability factors for the development of an anxiety disorder is mainly sex: women have twice the risk of developing an anxiety disorder, and the risk increases during adolescence (McLean et al., 2008). Another factor that is considered a risk for the development of an anxiety disorder is having parents with anxiety or depression; the heritable component has been studied, but remains undetermined (Shimada-Sugimoto et al., 2015). One of the most reported factors is stress in the early stages, such as abuse, parental neglect, and reduced social contact between children and their parents (Uliaszek et al., 2012). Finally, suffering from an anxiety disorder triples the risk of developing another anxiety disorder or doubles the risk of developing a depressive disorder (Lieb et al., 2002).
Depressive disorders are characterized by a lack of interest or pleasure, fatigue, persistent depressed mood, sleep problems, and sexual appetite (American Psychiatric Association, 2013). The relationship between stress and depressive disorders again lies in the similarity of the neural mechanisms that underlie both conditions, mainly the structures of the limbic system, hippocampus, amygdala, and prefrontal cortex (Roy & Roy, 2017).
The vulnerability factors for the development of a depressive disorder involve exposure to stress in the early stages (Slavich et al., 2011), or during adulthood (Willner et al., 2013). Furthermore, the interaction between various genes and the environment (Caspi et al., 2003), as well as the preexistence of a depressive phenotype (Fogel et al., 2006), can be vulnerability factors. Changes possibly enhanced by stress obey the hypothesis of stress diathesis (Willner & Belzung, 2015). This hypothesis indicates that the predisposition (diathesis) to develop depression because of exposure to stress can occur due to genetic predisposition and exposure to stress at different stages of life. The amount of stress required to trigger the disorder will depend on the individual’s diathesis and their ability to cope with stress (Willner, 2017). Finally, anxiety disorders and depressive disorders have high comorbidity, sometimes anxiety first, then depression or vice versa; the relationship suggests that neurobiological alterations are shared between both disorders (Shin & Liberzon, 2010).
11. Conclusion
The study of the stress response, its implications, and consequences is very important to understand its relationship with the beginning of different psychiatric disorders (Trucco, 2002). The interplay between stress and life stages highlights the importance of allostatic load and the impact of experience in the development of psychiatric illnesses. Although its conceptualization has not been defined, the similarity in the definition proposals allows us to rescue important aspects to consider when it comes to stress. On the other hand, the description of its neurobiology is well-studied and shows evidence of the possible mechanisms that are involved in its functioning and dysregulation (McEwen, 2000). Even though the stress response involves central systems, such as the limbic system and the HPA axis, it also makes use of peripheral systems as the SAM axis, which is part of the ANS (de Kloet et al., 2005). On the other hand, the release of CORT by the neuroendocrine response has been studied with great attention; its genomic and non-genomic physiological activity, the rhythmicity of its release, and the regulatory role of the stress response are the most common topics in its study.
Finally, an aspect not considered in the study of stress is the regulation or modulation of cognition, both for the triggering of the response and the termination and its therapeutic use. The unpredictability and the feeling of loss of control stand out within this topic because they worsen or increase the negative effects of exposure to stress. Likewise, the stress response changes depending on the stage of life in which we find ourselves, from the fetal stage to old age, the behavioral dynamics of the HPA axis are different, and the response may be imprecise and increased during the early stages of the development as the latest. Finally, the altered CORT response, a product of exposure to stress, is of utmost importance to understand the changes associated with the triggering of various psychiatric disorders such as anxiety and depression (Doczy et al., 2009). These are mainly because they share neuronal bases associated with the limbic system, the prefrontal cortex, and the brain stem, structures that have a high expression of GR and MR receptors.