INTRODUCTION
The Piper genus has 291 species, of which 184 are endemic to Brazil (Flora do Brasil, 2018). These species have been studied because of the production of essential oil with different biological activities (Pereira et al., 2008; Lopes et al., 2012).
Piper arboreum Aubl., known as "long pepper", "rosemary-angola", "fruit-of-bat", "jaborandi" and "jaborandifrom-river", is a perennial shrub with wide distribution, especially in the dense ombrophylous segment of Atlantic Forests (Guimaraes et al., 2006).
Several biological activities in P. arboreum result from the essential oil composition, such as a leishmanicidal action attributed to sesquiterpenes (Bernuci et al., 2016). The main constituint, sesquiterpene bicyclo-germacrene, was reported in plants from the Federal District area by Potzernheim et al. (2006), whereas the oxygenated sesquiterpenes 1-epi-Cubenol, Spa-thulenol, α-Cadinol and epi- α-Muurolol were observed in plants collected from Antonina-PR (Bernuci et al., 2016).
Piper cernuum Vell., known as "pariparoba", is a native non-endemic species found in primary and secondary forests in Atlantic Tropical Areas (Flora do Brazil, 2018), which has been used in traditional medicine for stomach, liver, kidney and circulatory treatments (Mariot et al., 2007).
The essential oil produced by this species has the monoterpenes α-pinene and β-pinene and the oxygenated sesquiterpenes spathulenol, epi-α-muurolol, α-muurolol, caryophyllene oxide and α-cadinol, which have shown high leishmanicidal activity and moderate anti M. tuberculosis activity (Bernuci et al., 2016). The sesquiterpenes dihydro-β-agarofuran and 10-epi-y-eudesmol, besides the monoterpenes a-pine-ne and camphene, were the main constituents and have shown antibacterial activity (Perigo et al., 2016).
Furthermore, Girola et al. (2015) verified induced apoptosis in melanoma cells by a camphene compound isolated from P. cernuum essential oil.
Piper diospyrifolium Kunth., a semi-heliophilous perennial shrub regionally known as "joao-borandi", is found in humid areas of dense low montain forests and in lowland forests in Rio de Janeiro, São Paulo and Parana (Brazil) (Guimaraes et al., 2006). The essential oil composition presented the sesquiterpenes selin-11-en-4-a-ol, germacrene B and a-selinene, with inhibitory growth potential of Leishmania forms (Perigo et al., 2016). Sesquiterpenes were also related to antifungal potential against Candida species (Vieira et al., 2011).
These species have a common characteristic, the high environmental regulation of essential oil production (Bergo, 2010; Oliveira et al., 2013; Silva et al., 2013; Perigo et al., 2016), which results in differences in yield and composition according to the plant collection location. Even in the same location, high variability can be found in the essential oil production of Piper species because of natural propagation through seeds. The development of vegetative protocols could be very useful for these species in order to maintain the essential oil yield and quality of selected plant materials for cultivation.
Some vegetative propagation protocols have been developed for Piper using different types of propagules (Cunha et al., 2015; Gomes and Krinski, 2016a), substrates, luminosity and seasonality (Mesquita et al., 2005; Dousseau, 2009; Gomes and Krinski, 2016b; Pacheco et al., 2016; Gasparetto et al., 2017). However, there is a lack of information on propagation for the great majority of species, especially for the three species included in this study.
The objective of this study was to evaluate the root production of Piper arboreum, P cernuum and P diospyrifolium after treatment with indole butyric acid to develop cultivation protocols and reduce extractivism.
MATERIALS AND METHODS
The botanical specimens used in this study were collected in April, 2016 in the "Biological Reserve Bom Jesus", Parana state (Brazil), located at 25°29'69.3'' S and 49°00'84.4'' W, at sea level. Samples of the botanical species were deposited in the herbarium of the "Botanical Museum", located in the "Botanic Garden of Curitiba" under the numbers MBM 396412 (Piper arboreum Aubl.), MBM 396416 (Piper cernuum Vell.) and MBM 396413 (Piper diospyrifolium Kunth.).
The plant material was collected and moistened in the field until the stem cutting preparation. Cuttings with a length between 10-12±2 cm and a diameter of 6±2 mm were prepared maintaining one pair of leaves with half of the leaf area. Before the rooting treatments, the stem cuttings were kept in running water for 5 min.

Figure 1 Semihardwood stems from A. P. arboretum; B. P. cernuum; and C. P. diospyrifolium (Ferriani, 2016).
This experiment was conducted with a completely randomized design for each species, comparing five treatments [immersion in hydro alcoholic solutions containing 0, 500; 1,000; 1,500; 2,000 and 3,000 mg L-1 of indole butyric acid (IBA) for 10 s], with four replications with 10 cuttings each. After the treatment, the stem cuttings were transferred to polypropylene tubes (53 cm3) containing the commercial substrate Tropstrato HP® maintained in a greenhouse with intermittent misting, relative humidity around 80% and temperatures ranging for 20-30°C for 90 d.
The rooting percentages, number and average length of the three main roots, foliar retention, sprout and shoot mortality were evaluated. The data was submitted to homogeneity analysis with Bartlett's test and the analysis of variance and regression using the statistical software Assistat (Silva et al., 2016).
RESULTS AND DISCUSSION
In all analyzed species, the rooting response were positively affected by the plant growth regulator indole butyric acid. P. arboreum reached 70% rooting percentage of cuttings when the highest IBA concentration was used (Fig. 2).
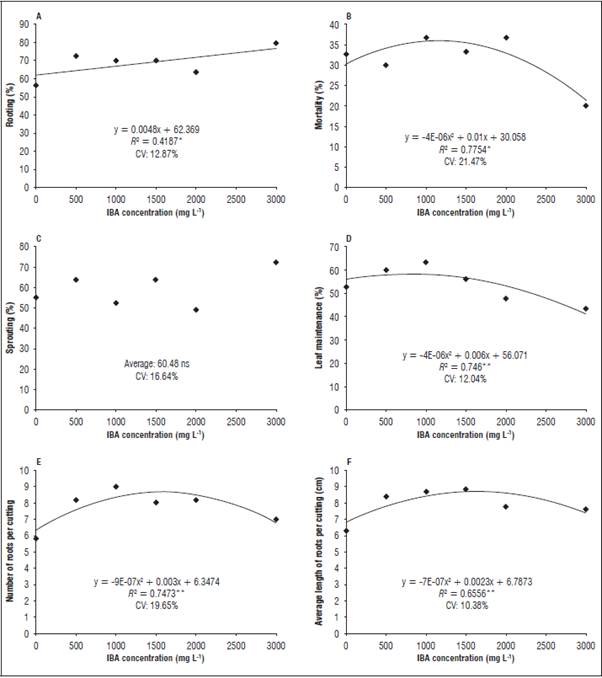
Figure 2 Rooting percentage, mortality, sprouting, leaf retention, number and average length of roots in the P. arboretum cuttings. **Significant at 1% probability according to polynomial regression analysis; *significant at 5% probability according to polynomial regression analysis. ns: not significant. CV: coefficient of variation.
Variables related to rooting response, such as leaf retention percentages, number and length of roots were negatively affected by the IBA concentrations over 1,500 mg L-1, which may indicate a phytotoxicity effect. These results may be related to the intrinsic capacity of natural vegetative propagation with stolons, as pointed by Souza et al. (2009).
In this species, leaf loss at high concentrations of an exogenous regulator affect adventitious rooting because mature leaves act as a source of carbohydrates and cofactors as verified in P. hispidum, which presented higher numbers of sprouts and roots in cuttings that maintained a higher number of leaves (Cunha et al., 2015). The rooting of the P. cernuum cuttings showed an upward behavior with a maximum of 45% in 3,000 mg L-1 of IBA, and it was possible to verify that the highest concentrations also promoted the highest percentages of this variable, limited by the plant regulator treatments (Fig. 3).
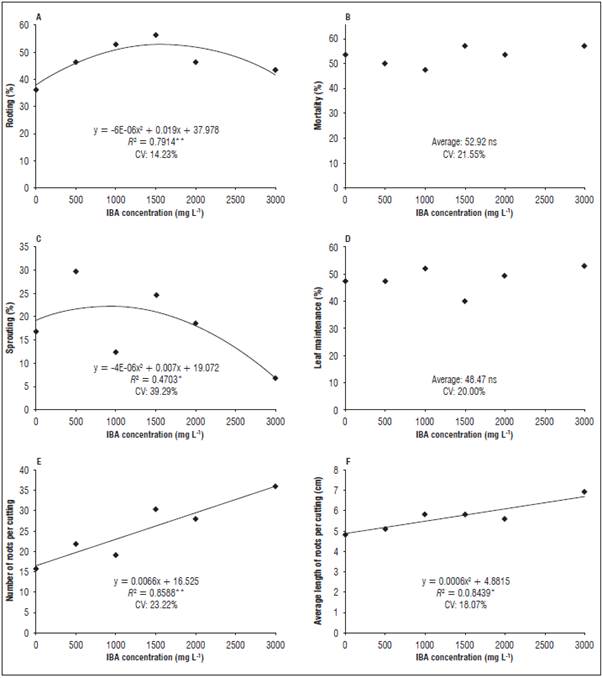
Figure 3 Rooting percentage, mortality, sprouting, leaf retention, number and average length of roots in the P. cernuum cuttings. **Significant at 1% probability according to polynomial regression analysis; *Significant at 5% probability according to polynomial regression analysis. ns: not significant. CV: coefficient of variation.
The percentage of sprout shoots showed a linear increase as a function of the higher IBA concentration. It was not possible to associate the behavior of the leaf retention variable to any treatment whose overall mean was 25.4%. However, for the number and root length variables, it was possible to verify the tendency to increase the values up to the limit of 2,000 mg L-1. These results suggest that doses containing higher concentrations may trigger phytotoxic effects, leading to a reduction of the quality of the adventitious root system.
In comparison with other species from the same family, Piper cernuum presents a greater leaf area (almost five times greater), which represents higher metabolic intakes and losses. Thus, in this case, the leaf loss was associated with an increase in the percentage of shoots that promoted the translocation of photoassimilates to a critical level, from which there was a reduction in the number and length of the roots.
The P. diospyrifolium cuttings reached a percentage of rooting of 70%, being influenced by the application of IBA treatments up to the concentration of 1,500 mg L-1, since there was a reduction in the means of this variable with the higher IBA concentrations (Fig. 4).
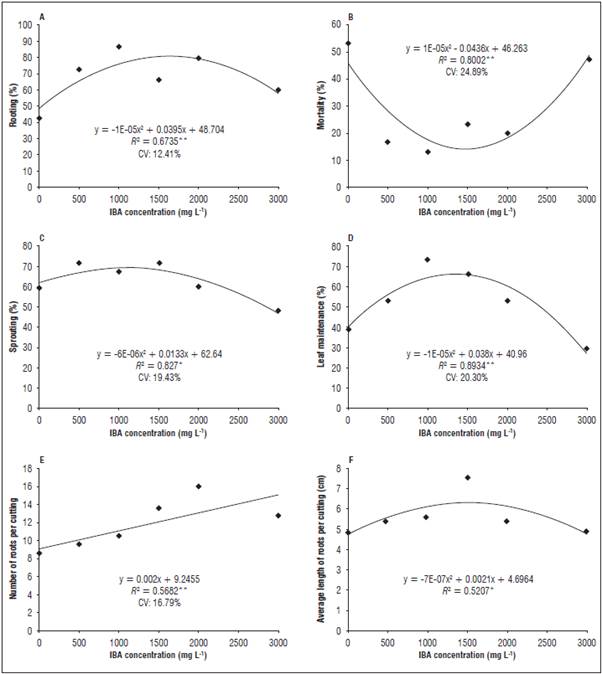
Figure 4 Rooting percentage, mortality, sprouting, leaf retention, number and average length of roots in the P. diospyrifolium cuttings. **Significant at 1% probability according to polynomial regression analysis; *Significant at 5% probability according to polynomial regression analysis. ns: not significant. CV: coefficient of variation.
The percentages of sprouts and leaf retention indicate that high doses of the plant regulator may have caused foliar abscission, interfering in propagule sprouting and, consequently, in root induction and the root length variable. Vignolo et al. (2014) verified a similar behavior for the cuttings of three blackberry (Rubus spp.) cultivars, where the presence of leaves positively affected the sprouting and rooting in all of the evaluated cultivars.
The number of roots varied linearly as a function of the plant regulator treatments. In Piper mikanianum,Pescador et al. (2007) related an increase in the rooting rate with the foliar maintenance and sprout formation in the cuttings. According to Hartmann et al. (2002), mature leaves and sprouting development increase the synthesis of cofactors related to the rooting process.
These results show the importance of the specific evaluation of rooting production for each Piper species since they present different responses in root induction and development.
The formation of an adventitious rooting system is dependent on the endogenous level of hormones and other rooting promoters (Hartmann et al., 2002). In P. hispidum for example, the rooting of stem cuttings reached percentages greater than 80% without the application of exogenous auxins (Cunha et al., 2015), and stem cuttings from P. amalago reached only 22% under the same auxin concentration (Gomes and Krinski, 2016a).
Other studies that consider the seasons for plant collection are recommended for the root production of these species.
The present study provides unprecedented information on the propagation of these Piper species, which will help establish protocols that guarantee sustainable cultivation practices since the current use of raw material is exclusively obtained with extractivism.
CONCLUSIONS
All of the evaluated species presented an increase in root production after treatment with indole butyric acid. The rooting percentages reached averages of 67.5% for P. arboreum, 51.6% for P. cernuum and 50.4% for P. diospyrifolium.
These positive results show the importance of specific studies on the domestication of Piper native species for crops development.















