Yellow diploid potato o potato criolla (Solatium tuberosum L. Group Phureja) corresponds to the diploid morphotypes, with tubers with a yellow skin and flesh color (egg yolk phenotype) (Rodríguez et al., 2009). Colombia is the main commercial producer, largest consumer and exporter of diploid potatoes in the world, with the competitive advantages of being the center of diversity and wide acceptance among consumers because of its organoleptic characteristics, which makes the potato criolla one of the genetic resources of high importance in the country (Herrera and Rodríguez, 2011). For 2016, approximately 10,683 ha of this crop were planted, for an annual production of 170,000 t. The Cundinamarca, Nariño, and Boyaca provinces have the highest production (Agronet, 2016).
INTRODUCTION
Boron phytotoxicity is observed with the presence of chlorosis and necrosis in the tips and margins of the leaves, with subsequent intermittent burning; it starts in mature leaves, and chlorosis and necrotic margins could be evidenced under severe toxicity conditions in the leaves of the medium strata (Herrera et al., 2010; Metwally et al., 2012). This reflects the poor mobility of B through phloem in Solanaceae species (Di Gioia et al., 2017), which could be influenced by the transpiration rate and other factors (Brown and Hu, 1998; Eichert and Goldbach, 2010).
Plants absorb B in the form of anion borate B(OH)4- or boric acid B(OH)3 (Brown and Hu, 1998). One of the primary functions of B in plants is related to the structure of cell walls (Herrera et al., 2010; Metwally et al., 2012), where B integrates rhamnogalacturonan II of pectin and promotes growth, cell wall rigidity, and membrane stability (Archana and Verma, 2017). However, an excess of B could produce membrane alterations and oxidative damage through lipid per-oxidation with excessive accumulation of hydrogen peroxide (Herrera et al., 2010; Reid, 2010). B toxicity in tomatoes results in increased activity of antioxidant enzymes derived from the ascorbate cycle (Cervilla et al., 2012); in addition, B toxicity causes an increase in levels of malondialdehyde in potatoes (Ayvaz et al., 2013) and a release of electrolytes in radish cells (Siddiqui et al., 2013). These parameters are used as indicators of B toxicity affecting cell membranes (Mohammed et al., 2002; Barrett and Douglass, 2004; Siddiqui et al., 2013).
Another stress response as a result of B toxicity in plants has been observed with the biosynthesis of non-enzymatic antioxidants, such as glutathione and cysteine, in sunflowers (Ruiz et al., 2003) and with different pathways of proline synthesis in rice, depending on the intensity of the B stress (Dominic and Jithin, 2012); the accumulation of proline in Solanaceae has been also observed, such as in tomatoes (Solanum lycopersicum) (Cervilla et al., 2012). Proline accumulates in the cytosol and allows an osmotic adjustment, which stabilizes subcellular structures, regulates redox potential, and blocks free radical production under excess B (Bonilla and González, 2011; Cervilla et al., 2012).
In Colombia, B is applied with foliar spraying on yellow diploid potatoes with variable doses, without knowledge on toxicity or physiological and metabolic responses for tetraborate sodium and boric acid although it is presumed from field experiences that a B source accompanied by Na could be more toxic than boric acid, which is related to absorption and mobility conditions as well as toxic effects potentiated by the presence of Na (Dominic and Jithin, 2012). On the other hand, foliar B applications have been shown to be an effective alternative in mineral nutrition with this element, where periodic applications should be made during the growth phase to avoid B toxicity resulting from possible accumulation of B in plants because of its low mobility (Fernández et al., 2013).
The management of foliar B applications under conditions of B deficiency or low availability favors production with B doses that can vary between 400 to 1000 g ha-1 per cycle depending on the species (Castro and Gómez, 2010; Fernández et al., 2013).
The objective of this study was to determine the effects of different boron supply treatments and the effects of foliar application of B with sources of high solubility (sodium borate and boric acid) on changes in growth and physiological parameters in yellow diploid cv. Criolla Galeras.
MATERIALS AND METHODS
Plant material and location
The experimental phase was carried out in the first semester of 2013 in the greenhouses of the Faculty of Agricultural Sciences, of the Universidad Nacional de Colombia, Bogota characterized by an average PAR of 393.07 /xmol m-2 s-1, average air temperature of 26.3°C, and average air humidity of 58.5%. Tubers of yellow diploid potato cv. Criolla Galeras of a homogeneous size (3-4 cm) were planted individually in plastic bags with 1.5 kg of soil. After 30 days of planting (DAP), a balanced edaphic maintenance fertilization was carried out according to the soil chemical analysis. The soil was classified as Typic Hapludand with average contents of B (<0.6 mg kg-1, extraction by monobasic phosphate). The measurements of the plant response to the B applications were done 90 DAP.
Experimental design and treatments
Four foliar applications of B were carried out during the study, distributing the total dose during growth (Tab. 1). Each spraying was done with surfactant adjuvant (1 ml L-1 Transfer® Adhex) at an interval of 4 d, starting at 45 DAP using 13 ml of water per plant per application, which corresponded to 400 L ha-1 (average application volume in the field). A completely randomized design was used with nine treatments (Tab. 1) and four replicates; each replicate corresponded to one plant. An analysis of variance was performed, and Tukey's mean comparison test (P<0.05) between the treatments was applied using Infostat® (National University of Cordoba, Argentina). Additionally, Sperman correlation analysis was used. To determine the response of the interaction between the sources and doses, a 2x4 factorial analysis was carried out for each of the variables, where the main factor was the B sources (boric acid, sodium borate), and the secondary factor was the B doses (0.5, 1.0, 2.0, or 4.0 kg ha-1 per cycle).
Table 1 Treatments and doses of boron applied via foliar on yellow diploid potato cv. Criolla Galeras
| Sources | |||||||
| kg ha-1* | mM | g L-1 ** | kg ha-1*** | g L-1* | g L-1** | ||
| 1 | Boric acid (17.5% B) | 0.5 | 28.6 | 0.31 | 2.86 | 7.14 | 1.79 |
| 2 | 1.0 | 58.2 | 0.63 | 5.71 | 14.29 | 3.57 | |
| 3 | 2.0 | 115.6 | 1.25 | 11.43 | 28.57 | 7.14 | |
| 4 | 4.0 | 231.2 | 2.50 | 22.86 | 57.14 | 14.29 | |
| 5 | Sodium borate (20.5% B) | 0.5 | 28.6 | 0.31 | 2.44 | 6.10 | 1.52 |
| 6 | 1.0 | 58.2 | 0.63 | 4.88 | 12.20 | 3.05 | |
| 7 | 2.0 | 115.6 | 1.25 | 9.76 | 24.39 | 6.10 | |
| 8 | 4.0 | 231.2 | 2.50 | 19.51 | 48.78 | 12.20 | |
| 9 | Control | 0.0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Total dose in crop cycle. ** Individual dose in each of four applications. *** Volume of water 400 L ha-1 in each of four applications.
Variables evaluated
Growth variables. At 90 DAP, a destructive analysis of the aerial parts of the plants was performed. The fresh weight of the leaves and stems of each plant was determined, and the leaf area (LA) (cm2) was quantified with a Li-3100 (LI- COR Inc., USA). The dry weight of the organs was determined after drying in an oven at 70 °C for 72 h. With the dry weight of the aerial part (DW) and LA, the growth physiological index specific leaf area (SLA) was determined (Gardner et al., 2003).
Electrolyte leakage (EC). For the measurements, 0.5-1 cm3 leaf segments were taken from the middle-third of the plants to determine the loss of electrolytes in the leaves (ECh). To measure the loss of electrolytes in the stem (ECt), 1 cm long parts of the main stems were taken. The method of Mohammed et al. (2002) and Barrett and Douglass (2004) was employed to measure the electrical conductivity of the solutions at 30°C (EC30 °C) and 100°C (EC100 °C). The percentage of electrolyte leakage from the cells was determined with (1):
EC (%) = (EC30 °C / EC100 °C) X 100% (1)
Proline contents. Fresh plant material of the leaves from the middle-third of the plants was used. Absorbance at À=520 nm was measured with a spectrophotometer (Spectronic® 501, Milton Roy, Rochester, NY) using toluene as the blank (adapted from Bates et al. (1973)). The proline concentration was determined on a fresh weight (FW) basis with (2):
Proline contents (μM) / g FW = ((μg proline / mL) x mL toluene) / (115.5 /μg /μM) (2)
RESULTS AND DISCUSSION
Growth variables
The dry mass of the aerial part had the highest response for the dose of 1.0 kg ha-1 B as Na borate with a 15.85% relative increase in DW (Fig. 1), and, for boric acid, a 25.1% relative increase in DW was obtained with respect to non-foliar application of B, which is consistent with that reported by Asad et al. (2002). Significant differences (P<0.01) were found between the B sources for DW LA, and SLA (Figs. 1-3), with a lesser toxic effect of B on DW for boric acid, as compared to Na borate at doses higher than 2 kg ha-1 (Fig. 1); these concentrations of B as Na borate reduced the DW by around 25% with respect to the non-application of B (Fig. 1). This phenomenon can be explained by the interaction between B and Na, which potentiates salinity stress and slows down transpiration and growth processes in a more restrictive way, reaffirming the findings of Bonilla and González (2011) and Dominic and Jithin (2012).
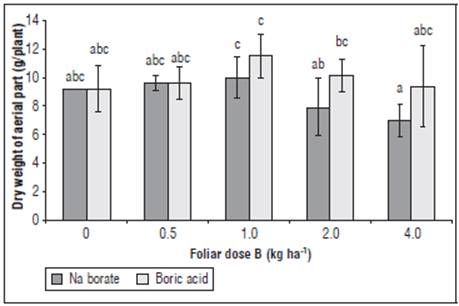
Figure 1 Effect of foliar application of different doses and sources of B on dry mass of aerial parts (DW) of yellow diploid potato cv. Criolla Galeras. Means with different letters indicate a significant difference according to Tukey's test (P<0.05). The bars correspond to standard deviation.
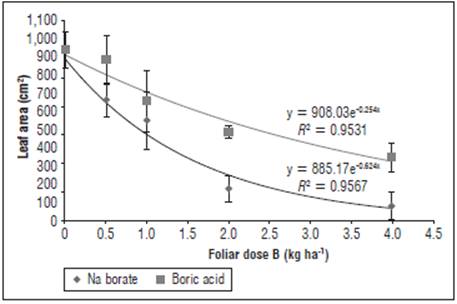
Figure 2 Effect of foliar application of different doses and sources of B on the leaf area (LA) of yellow diploid potato cv. Criolla Galeras. The bars correspond to standard deviation.
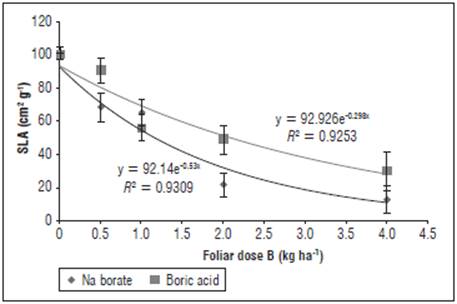
Figure 3 Effect of foliar application of different doses and sources of B on the specific leaf area (SLA) of yellow diploid potato cv. Criolla Galeras. The bars correspond to standard deviation.
The lower impact of B stress on the DW at doses higher than 2 kg ha-1 of boric acid could be explained by the lower accumulation of B in tissues, the relative increase in B mobility, and the absence of Na in the source since boric acid penetrates the double layer of membrane phospholipids through simple diffusion and is transported by B-polyol complexes that allow bidirectional movement of B, with distribution in organs with a low concentration of B, as observed by Reid (2010). This lower impact of boric acid toxicity on DW was also reported by Karabal et al. (2003) in barley, but contradicts the results of Reid (2010), who suggested that B toxicity is not completely explained by an osmotic stress of B, but also by an interaction with other synergistic abiotic factors, such as photo-oxidation and other synergistic ions.
The highest DW was seen with the B dose 1 kg ha-1 cycle-1 for both sources of B, which evidenced the positive effect of foliar fertilization with B on the cultivation of yellow diploid potato cv. Criolla Galeras and confirmed the results of García and Poveda (2014), who obtained positive responses in DW when applying 0.5-1.0 kg ha-1 of foliar B as boric acid in cv. Criolla Colombia. The responses to doses of foliar B in Group Phureja were lower than those caused by edaphic B applications, where an increase in DW has been found with doses of 1.5 kg ha-1 in cvs. Yema de Huevo and Criolla Colombia (Pérez et al., 2008). These positive responses in growth coincided with the beneficial effects of B on productivity in several crops (Fernández et al., 2013; Archana and Verma, 2017), indicating the positive physiological functions of B in the elongation of new aerial organs, activating metabolic processes, such as the synthesis of nucleic acids, proteins, amino acids, starch, auxins, and phenolics, and transport of assimilates; however, B toxicity inhibits these functions (Cervilla et al., 2012; Shah et al., 2017).
The highest responses in LA and SLA of the plants were observed with doses between 0-0.5 kg ha-1 foliar B (Figs. 2 and 3); these B concentrations did not differ significantly (F<0.05) for the source of boric acid, but differed for the source of Na borate. In addition, significant differences were found for the doses higher than 2 kg ha-1 B between the sources, with a reduction in LA and SLA greater than 50%; whereas, with a dose of 4 kg ha-1 B, these reductions were about 65% for boric acid and about 90% for Na borate. This phenomenon can be explained by the reduction in leaf expansion in mature and new leaves, where toxic doses of B alter the structure of primary metabolites ATP, NADH, NADPH and the formation of bonds with RNA ribose, which limits RNA synthesis, affecting the formation of leaves and other organs, as reported by Reid et al. (2010) and Bonilla and González (2011). On the other hand, the decrease of LA at toxic doses of B (2 and 4 kg ha-1) in the cv. Criolla Galeras could be explained by abscission and death of old leaves, as observed in the field, where high concentrations of B might act as an inducer of abscisic acid synthesis (Bonilla and González, 2011; Macho et al., 2017). The restriction on growth by toxic levels of B was also observed in rice by Dominic and Jithin (2012), in citrus by Keles et al. (2004) and in tomatoes by Cervilla (2009); the latter report indicated decreases in LA and DW at toxic levels of 2 mM of edaphic B applications. In general, a lower LA before tuber filling affects the accumulation of DW and yield in S. tuberosum (Herrera and Rodríguez, 2011).
The toxic effect of B on growth could also be attributed to the fact that excess B reduces NO3 - conversion to NH4+ and, therefore, inhibits N metabolism and efficient formation of proteins as proposed Seth and Aery (2017). Additionally, high amounts of B in various crops decrease the growth of vegetative organs, chlorophyll contents, and photosynthetic rates as the result of osmotic imbalances and the inability to resist oxidative damage increases from photooxidation (Herrera et al., 2010).
The application of boric acid favored the growth rates for yellow diploid potato cv. Galeras, which resulted in the highest accumulation of DW and was related to a higher LA and SLA, with respect to the control and Na borate. Even when applied at high doses, boric acid (2 and 4 kg ha-1) showed significant differences (P<0.05) with respect to the highest toxicity exerted by Na borate (Figs. 2 and 3); apparently, this was because of the effects of Na ions and possible osmotic stress in the plants (Reid et al., 2004; Dominic and Jithin, 2012).
Plants that accumulate high levels of B can have mechanisms of B exclusion with guttation or accumulation in organs, such as the stem, and can present adequate growth in DW (Cervilla et al., 2012). These mechanisms might explain the highest effect of osmotic stress on the margins of the yellow dip-loid potato leaves of cv. Criolla Galeras because of accumulation of Na when used as an accompanying cation in sources of foliar B applications, with respect to boric acid, as was evidenced by the symptoms in field (Fig. 4). These results indicate a higher tolerance of the plants to B applied as boric acid and corroborate the positive responses of foliar boric acid applications, as described by Brown and Hu (1998) and Fernández et al. (2013). The results of the current study establish that yellow diploid potato cv. Criolla Galeras is tolerant to foliar applications of boric acid at doses of 0.5 to 2 kg ha-1 although it is important to evaluate the effect of B applications on tuberization and commercial production in future studies.
Metabolic responses
Highly significant differences (F<0.001) were observed in the ECh, with a linear response of this variable and a high coefficient of determination (R 2 > 0.96) with the increasing doses of B for both sources in cv. Criolla Galeras (Fig. 5). This coincides with the reports by Karabal et al. (2003) for barley and by Siddiqui et al. (2013) for the radish, who found higher percentages of ECh in leaves treated with B as the dose of B increased (5-10 mM) although at different proportions than in the results obtained for cv. Criolla Galeras in the current study and with differences obtained between the species. Different types of abiotic stress can have similar effects on plants, which could be related to alterations in the integrity of the cell membranes (Reid, 2010; Savic et al., 2012). B toxicity promotes the appearance of reactive oxygen species, which affect metabolic processes through the alteration of lipids and can cause cell death, promoting a greater exchange of cytosol ions with the outside of the cell (Reid et al., 2004; Cervilla et al., 2012); therefore, the measurement of free electrolytes becomes an indicator of the loss of membrane stability caused by osmotic stress (Karabal et al., 2003; Cha-Um et al., 2010).
Savic et al. (2012), under conditions of heat stress in S. tuberosum, reported an ECh of 30% in tolerant potato cultivars, such as Laura, and an ECh of 75% in sensitive cultivars, such as Desiree. For nutritional stress resulting from excess B, with the maximum dose of 4 kg ha-1, the cv. Criolla Galeras in the present study reached as ECh of 62% for Na borate, which presented significant differences (F<0.001) from the maximum dose of boric acid that resulted in higher stability in the membranes, with an ECh of 28%. This stress indicator corroborated a greater oxidative damage in membranes for Na borate, where a higher slope of the trend line was observed with respect to that of boric acid (Fig. 5). In addition, a greater EC was observed with the foliar doses of B, exceeding 2 kg ha-1 (Fig. 5), which coincides with the reduced values of the growth variables (DW, LA, SLA) and the greater increases in proline contents (Fig. 6), which behaves as a compatible osmolyte and avoids oxidative stress resulting from excess B, protecting membranes and proteins in the cells (Dominic and Jithin, 2012).
Excess B limits the accumulation of solutes in plants; in young leaves, cell turgor can be reduced, accompanied by leaf chlorosis and deformations; while, in mature leaves, the accumulation of B favors osmotic stress, with membrane instability and high ion mobility that affect new tissues (Reid et al., 2004; Dominic and Jithin, 2012). In comparison with the non-application of B, the B dose of 4 kg ha-1 resulted in a loss of six times more electrolytes for Na borate and three times more electrolytes for boric acid (Fig. 5). This proves that the B stress did not only depend on the dose, but also on the source; in this case, the interaction with the accompanying Na ion favored greater stress, confirming the report by Dominic and Jithin (2012).
For the doses 0.5 and 1.0 kg ha-1 per cycle of foliar B, no significant differences for the ECh were observed, with ECh losses of 10 and 20%, respectively (Fig. 5), signifying that, for these doses, the integrity of the membranes was maintained at tolerable and manageable levels for cv. Criolla Galeras, which corresponded to the highest accumulation of DW (Fig. 1) and contrasted with insignificant losses of stem-free electrolytes (ECt) (data not shown). The ECh turned out to be a more reliable measurement in the evaluation of the EC; while for ECt, in future research, relating concentrations of the free ions B, K and Na in the leaf and stem sap is recommended in order to establish a better comparison.
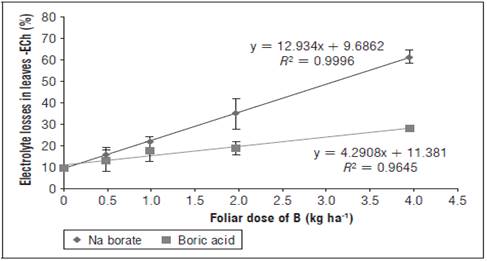
Figure 5 Effect of foliar application of different doses and sources of B on electrolyte loss (%) in leaves (ECh) of yellow diploid potato cv. Criolla Galeras. The bars correspond to standard deviation.
After the application of different concentrations of B in yellow diploid potato cv. Criolla Galeras, treatments with both sources increased the proline concentrations, with significant differences with respect to the control and differences between the sources. Less proline was accumulated after the foliar treatments with boric acid (Fig. 6), a source that did not cause significant differences (P<0.05) in the proline dynamics because of B toxicity in barley according to Karabal et al. (2003), while Na could increase proline levels in rice via the Ornithine route instead of the Glutamate route (Dominic and Jithin, 2012). On the other hand, Siddiqui et al. (2013) found that the radish increased proline levels when B toxicity was mitigated by Ca. Levy, and Veilleux (2007) and Cervilla et al. (2012) revealed an increase of this amino acid in potato and tomato plants subjected to B toxicity.
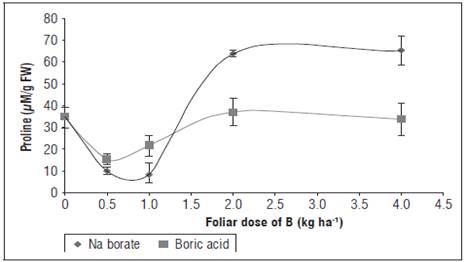
Figure 6 Effect of foliar application of different doses and sources of B on proline contents (pM/g fresh weight) in yellow diploid potato cv. Criolla Galeras leaves. The bars correspond to standard deviation.
Proline could be accumulated in cells as a result of an increase in its biosynthesis or alteration in the synthesis/degradation of proteins (Cervilla et al., 2012, Dominic and Jithin, 2012). This leads to the conclusion that, for the doses 0.5 and 1.0 kg ha-1 of foliar B, the cv. Criolla Galeras plants presented lower concentrations of proline than the control treatment, probably because of a reduced biosynthesis of proline since these were the treatments with lower stress (Fig. 6), which indicated better metabolic conditions related to a higher accumulation of DW and lower permeability of membranes, as discussed above. The doses greater than 1.0 kg ha-1 of foliar B significantly increased the concentrations of proline in the leaves (Fig. 6), implying an increase in membrane degradation and proteins, which were related to the highest ECh, which reached the highest levels at doses of 2 and 4 kg ha-1 B. Cervilla (2009) found that, in mature tomato leaves, an increase in proline as a result of excess B was inversely proportional to biomass accumulation and was the result of the inadequate metabolism of NO3 - reduction (Cervilla et al., 2012). These data suggest that, regardless of its protective role, an increase in proline concentration is another indicator of the stress produced by B toxicity in potato cv. Criolla Galeras.
The evaluated variables responded better to the treatments with boric acid than to the ones with Na borate. This might have been due to this source having better mobility in short- and long-distance transport, avoiding accumulation in the leaves, and being distributed to sources with the formation of complex esters or lignified structures in cell walls, corroborating the proposal by Herrera et al. (2010), in such a way that, under conditions with high concentrations of B, this type of bond is stronger than free B, which can be a detoxification mechanism for this mineral nutrient in some species.
CONCLUSIONS
In the potatoes, a possible osmotic stress, as indicated by the increased proline contents, was generated with B doses higher than 1.0 kg ha-1 , causing oxidative stress with irreversible damage related to decreases in the growth variables DW, LA, and SLA and a significant increase in ECh. ECh and proline contents in leaves could be used as stress indicators as a result of B in the yellow diploid potato cv. Criolla Galeras.
The lower impact of B stress exerted by the boric acid as compared to Na borate on the evaluated variables suggests that formulations or foliar applied products of B in potatoes should be based on boric acid. The symptoms of B toxicity were characterized for the yellow diploid potato cv. Criolla Galeras, presenting severe marginal and intervenal necrosis associated with chlorosis in leaves and, for new leaves, the reduction of growth, deformation of leaves, and marginal necrosis and generalized chlorosis. The results of this research could be used to search for good fertilization management practices with B in yellow diploid potatoes under the conditions of the Cundiboyacense highlands.
















