INTRODUCTION
The radish (Raphanus sativus L.) is a small vegetable that tolerates adverse conditions. Its origin lies in the Mediterranean region, and it belongs to the Brassicaceae family, which contains broccoli, cabbage, cauliflower and cabbage; however, the tuberous root is the part that is consumed, which has a reddish external color and a white flesh with a spicy flavor (Lanna et al., 2018).
Radish production has been stimulated because of the fact that it presents high rusticity with a short cycle crop, which means less time for harvesting, providing a fast economic return and making it a great alternative for intercropping with long cycle crops. It is appreciated for its vitamins A, B1, B2 and C, potassium, calcium, phosphorus and sulfur contents, conferring excellent medicinal properties, such as an antioxidant action, natural expectorant and coadjuvant of the digestive system (Oliveira et al. 2010; Cunha et al., 2017).
Radish crops are commonly irrigated, where the use of salt water is often the only alternative for production because quality water is increasingly scarce, especially in regions with high temperatures and low rainfall, resulting in increased salt concentration in the water (Lima et al., 2017). Among the abiotic stresses, saline is the most harmful to crop growth and productivity (Santos et al., 2012), reducing the osmotic potential of the soil solution, which can cause water deficits in plants and consequently reduce or inhibit the absorption of nutrients as a result of an ionic imbalance in the soil solution, causing phytotoxicity mainly through sodium and chloride ions (Eschemback et al., 2014).
In addition, saline stress can also cause plant dehydration as a result of a high salt uptake, meaning plants cannot compartmentalize and raising the concentration of these salts within plants (Parihar et al., 2015). In this sense, plants need greater energy expenditure to perform their vital functions, affecting growth, development and consequently productivity (Silva et al., 2012).
One of the mitigating methods for saline stress in plants is the application of ascorbic acid, which is an important antioxidant, protecting plants from oxidative stress, especially when caused by saline stress (Gul et al., 2015). It also helps the enzyme ascorbate peroxidase (APX) convert hydrogen peroxide (H2O2), which is a reactive oxygen species (EROS) produced in greater amounts in plants under stress that affects cellular homeostasis, playing a key role in tolerance to salinity and influencing stomatal opening, besides being easily absorbed by plants after foliar applications (Hameed et al., 2015).
In this context, it is necessary to establish the dose that provides the best attenuation results under saline stress in radish cultures. The aim of this study was to evaluate the effect of different electrical conductivities of irrigation water and ascorbic acid doses on agronomic performance and gaseous exchanges of the radish (R. sativus).
MATERIAL AND METHODS
This experiment was carried out from October to November, 2017, in a protected environment (greenhouse), in the Department of Plant Science and Environmental Sciences of the Center of Agricultural Sciences of the Federal University of Paraíba (UFPB), in the city of Areia, PB (Brazil). The soil used in the experiment was an Oxisol (Tab. 1), collected from a 0-20 cm depth layer.
Table 1 Results of the chemical soil analysis used in this experiment.
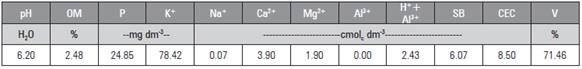
OM: organic matter; SB: sum of bases; CEC: cation exchange capacity; V: saturation by bases.
This experiment was carried out in a randomized complete block design, composite central box experiment (Bortoluzzi and Alvarez, 1997), in a 5x5 factorial scheme, with four replications for five doses of ascorbic acid (AA - 0.0, 0.29, 1.0, 1.71 and 2.0 mM) and five electrical conductivities in the irrigation water (ECw - 0.5, 1.3, 3.25, 5.2 and 6.0 dS m-1). A treatment that was irrigated with 0.5 dS m-1 water and no ascorbic acid was used to determine the irrigation depth using drainage lysimetry. The experiment plots were represented by plastic bag for the seedlings, with a capacity of 3.0 dm3. Margaret Queen Kobayashi hybrid radish seeds were used, sowing five seeds in each plastic bag at a depth of 2 cm. On the 15 day after sowing (DAS), thinning was carried out, leaving the plants more vigorous.
The lowest salinity water (0.5 dS m-1) came from the UFPB's supply system. A mixture of salts of NaCl, CaCl2-2H2O and MgCl2-6H2O and 0.5 dS m-1 water were added to the water with the highest salinity (1.3, 3.25, 5.2 and 6.0 dS m-1), maintaining an equivalent ratio of 7:2:1 (Medeiros, 1992), measuring the ECw with a portable conductivity meter. Throughout the experiment, the plants were irrigated with the ECw that was established for each treatment, with irrigation management done using drainage lisimetry.
Foliar applications of ascorbic acid were carried out at 19 DAS with the aid of an atomizer containing the doses of ascorbic acid and the adjuvant (Tween® 80) at a concentration of 0.0002% of the syrup.
At 35 DAS, the following variables were analyzed: leaf number (LN), counting all the leaves of each plant; stem diameter (SD), using a digital caliper; leaf area (LA), with the aid of a graduated ruler to record the length and width dimensions of the leaves and then the equation LA = C*L*f, from Benincasa (2003), where LA refers to leaf area; C leaf length in cm; L leaf width in cm; and f correction factor for radish = 0.57; specific leaf area (SLA), according to Witkowski and Lamont (1991), where SLA = leaf area * dry leaf weight; root dry mass (RDM) and tuber dry mass (TDM), determined on a scale with a precision of 0.001 g. In order to obtain the dry root and tuber masses, the material was packed in paper bags and oven dried at a temperature of 65°C until reaching constant weight.
The gaseous exchange determinations were carried out at 34 DAS, using an infrared gas analyzer (IRGA, LI-COR - model LI-6400XT Portable Photosynthesis System) from 9:00 am to 10:00 am. The following characteristics were measured: net CO2 assimilation (A - /xmol CO2 m-2 s-1); stomatal conductance (gs - mol H2O m-2 s-1); internal CO2 concentration (Ci - mmol CO2 m-2 s-1); transpiration rate (E - mmol H2O m-2 s-1); water use efficiency (WUE - A/E) and vapor pressure deficit (VPD - kPa).
Based on the accumulation of total dry matter and leaf area, the growth variables were determined according to Benincasa (2003), corresponding to the relative growth rate, RGR (g g-1 d-1), using the equation RGR = (LnW2 - LnW1) * (T2 - T1) -1, where LnW1 and LnW2 are the variation of the neperian logarithm of the dry mass between two periods, and T1 and T2 are the time variation between the periods. The leaf area ratio, LAR (cm2 g-1), was determined using the equation LAR = L * W-1, where L is the leaf area and W the total dry mass of the plant. The data obtained in the evaluations of the experiment were submitted to analysis of variance and regression using SAS University software (Cody, 2015).
RESULTS AND DISCUSSION
According to the analysis of variance, there was no interaction between the ascorbic acid (AA) and the electrical conductivities of the irrigation water (Ecw); also, there was no effect between the doses of ascorbic acid for both agronomic performance variables for the variables of gas exchange, probably because radish plants demand higher doses.
However, a difference was observed between the ECw applications in the radish plants. It was observed that, as the ECw increased (Fig. 1, 2 and 3), the values of the agronomic performance variables (leaf number, stem diameter, leaf area, specific leaf area, tuber fresh mass, root dry mass, relative growth rate and leaf area ratio) deceased.
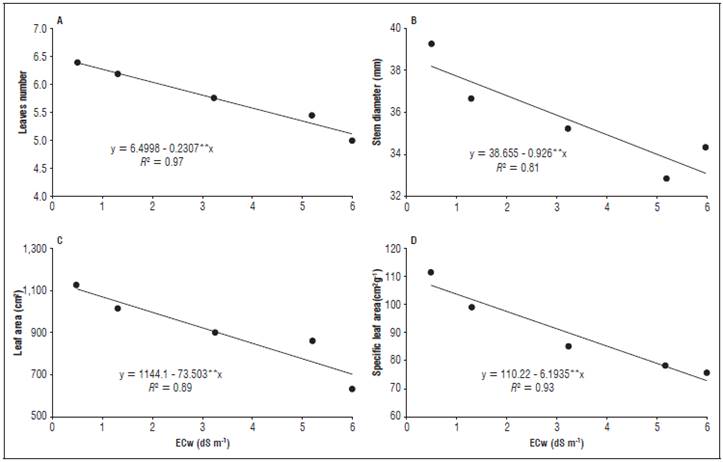
Figure 1 Leaf number (A), stem diameter (B), leaf area (C) and specific leaf area (D) of the radish plants as a function of the different electrical conductivities of the irrigation water (ECw).
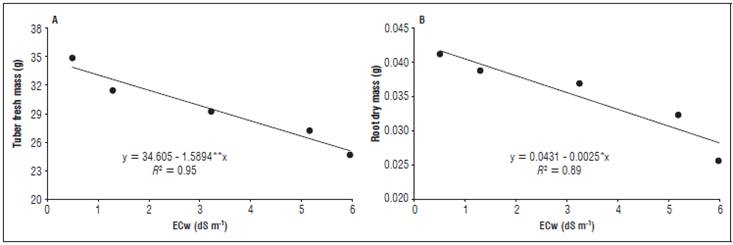
Figure 2 Fresh grass mass (A) and root dry mass (B) of the radish plants as a function of the different electrical conductivities of the irrigation water (ECw).
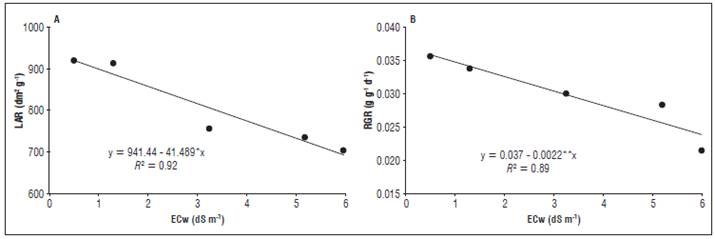
Figure 3 Leaf area ratio (A) and relative growth rate (B) of the radish plants as a function of the different electrical conductivities of the irrigation water (ECw).
Saline stress is the most damaging to crop growth and productivity among the abiotic stresses (Santos et al., 2012), causing reductions in the osmotic potential of the soil solution and consequently water deficits in plants, promoting a reduction or inhibition of the absorption of some nutrients as a result of an ionic imbalance of the soil solution and phytotoxicity, especially with sodium and chloride ions (Eschemback et al., 2014).
Several harmful effects caused by saline stress on plants can be related to the results obtained in the present study, where the higher salt concentrations intensified the negative reflexes in the development of the radish plants. Sousa et al. (2016a), evaluating the growth and productive performance of radish plants under different Ecw applications, observed results similar to those of the present study, where irrigation water with a higher electrical conductivity provided lower values for leaf area, shoot dry mass, fruit diameter, average fruit mass and productivity.
Given the variable number of leaves (Fig. 1A), it was notable that, in the lowest (0.5 dS m-1) and highest (6.0 dS m-1) Ecw applications, there were 6.4 and 5.1 leaves per plant, respectively, corresponding to a reduction of 20.3%. Similarly, Oliveira (2010), also working with salinity in a radish culture, obtained 6.9 leaves with the lowest Ecw application (0.5 dS m-1) and 3.6 leaves with 5.0 dS m-1, corresponding to a reduction of 47.6%, well above the present study even though the maximum ECw value was lower than that applied by this author. Osmotic imbalances, damage from oxygen reactive species (ROS) and ionic toxicity are the main characteristics observed in radish plants under saline stress (Sun et al., 2016).
As for stem diameter (Fig. 1B), a reduction of 13.4% was observed, with the respective values of 38.2 and 33.1 mm for the ECw amplitude (0.5 and 6.0 dS m-1). Santos et al. (2016), evaluating the growth and phyto-mass of beet plants under different ECw applications (1.0 to 5.0 dS m-1), observed that, up to an ECw of 3.0 dS m-1, the stem diameter increased, with a maximum value corresponding to 33 mm, with a decrease in stem diameter values beyond this ECw value.
The leaf area of the radish plants (Fig. 1C) varied negatively between the lowest and highest ECw applications (0.5 and 6.0 dS m-1), and the respective values were 1107.4 and 703.1 cm2, corresponding to a reduction of 36.5%. Oliveira et al. (2012) reported that an ECw of 2.0 dS m-1 resulted in the highest leaf area value (497.2 cm2), while the highest ECw (10.0 dS m-1) provided the lowest leaf area value (220 cm2), corresponding to a decrease of 55.75% for the radish culture.
When evaluating the growth and productivity of radish plants under different irrigation water salinities, 0.8, 1.5, 3.0 and 4.5 dS m-1, Sousa et al. (2016a) obtained a leaf area of 127 and 64.1 cm2 in the smallest and largest ECw applications, corresponding to a decrease of 49.5% in the leaf area, well above that of the present study. Srivastava et al. (2016), working with two varieties of radish plants under different concentrations of NaCl (0, 50, 100, 150 and 200 mM), also observed the same tendency for the variables leaf area, root and stem length and total biomass, where the higher salinity water provided lower values.
Saline stress alters the osmotic potential of the soil solution, which can cause water deficits in plants, which consequently reduce nutrient absorption, necessitating a higher energy expenditure and affecting growth, development and productivity (Silva et al., 2012). The results of this study evidenced a strong relationship, where the negative effect of one variable interfered, directly and negatively, with the others, explaining their similar behavior.
The highest leaf area (1107.35 cm2 g-1) was observed in the lowest ECw application (0.5 dS m-1), and the lowest specific leaf area (703.08 cm2 g-1) was seen in the highest ECw application (6.0 dS m-1), corresponding to a decrease of 36.5%. This negative effect was due to the inhibition of the development of the plants in the higher salt concentrations, reducing the leaf area and dry mass. Santos et al. (2017) also observed a decreasing behavior for specific leaf area in radish plants.
The tuber fresh mass and the root dry mass (Fig. 2A and B) also presented an inversely proportional behavior to the increase in the ECw applications, where the extreme results were 33.81 and 25.07 g for tuber fresh mass and s of 0.042 and 0.028 g for dry matter mas. for the lowest and highest ECw applications (0.5 and 6.0 dS m-1), corresponding to reductions of 74.15 and 66.67%, respectively. The same behavior was observed by Brunes et al. (2013) who worked with two rice cultivars, showing a decrease in the dry mass of the roots and shoots as the ECw increased.
Sousa et al. (2016a), studying salinity in a radish culture, observed a fresh mass of 9.11 and 2.13 g respectively for an ECw of 0.8 and 4.5 dS m-1, values well below those of the present study, but with similar behaviors and percentages of reduction (76.62%). Similarly, Oliveira et al. (2010), in a similar study, obtained inferior results for tuber fresh mass (19.07 and 9.12 g) using water conductivity (ECw) that varied between 0.5 and 5.0 dS m-1 in the radish culture.
These results were due to the high absorption of salts, which the plants could not compartmentalize, increasing the concentration of these salts in the interior and causing dehydration and consequently a reduction of the fresh mass (Parihar et al., 2015). According to Mekawy et al. (2015), another possible explanation would be the different responses of the evaluated cultivars since each genetic material has different tolerances and mechanisms under conditions of saline stress, where the one used in the present study stood out for these authors.
The leaf area ratio (Fig. 3A) was also affected by the increasing salinity, where the highest and lowest values (920.70 and 692.51 dm2 g-1) were obtained with the ECw extremes (0.5 and 6.0 dS m-1), as well as the relative growth rate (0.036 and 0.024 g g-1 d-1)(Fig. 3B).
Santos et al. (2014) observed the same behavior using irrigation waters with an ECw up to 4.0 dS m-1 in leguminous species, where waters with a higher ECw yielded lower results for the relative growth rate. The ECw increase also negatively affected the stomatal conductance (gs), the internal CO2 concentration (Ci) and the transpiration (E) at an ECw of 3.88, 4.34 and 4.30 dS m-1, respectively, with a subsequent increase in these variables at an ECw of 6.0 dS m-1 (Fig. 4). The reductions of these variables corresponded to 150, 20.3 and 60.6%, respectively. The net photosynthesis showed no significant effect; therefore, it was not shown in the graphs.
Such results can be justified by the presence of salts in the soil solution, reducing the water absorption capacity of the plants and causing water stress as the saline concentration increased because, in this situation, the main defense mechanism of the plants includes A, gs, Ci and E (Parihar et al., 2015), which is why these variables are directly related and presented similar behaviors, negatively affecting the photo-synthetic apparatus of the radish and, consequently, yield.
Ayyub et al. (2016), evaluating different radish genotypes under saline stress, emphasized that stomatal conductance decreased with an increasing salinity, where a lower stomatal condutace was observed with a conductivity of 7 dS m-1 and that the main effect of salinity is a reduction of photosynthetic processes. Sousa et al. (2016b), evaluating gaseous exchanges in citrus irrigated with saline waters, observed a reduction of gs, A, E and internal efficiency of carboxylation as the ECw increased from 0.6 to 3.0 dS m-1. Plants under saline stress also present a reduction of Ci because stomatal closure causes a decrease in the rate of assimilation of carbon in the leaf mesophyll (Santos and Brito, 2016). Ci is very relevant because the productivity of a plant can be understood as the product of interception of solar energy and fixed CO2, where an adequate amount of light and higher concentrations of CO2 provide higher photosynthetic rates. The stomatal closure impairs this activity, but, on the other hand, it reduces transpiration, reducing water loss in plants maintaining cellular turgescence and increasing photosynthetic efficiency (Taiz et al., 2017).
The vapor pressure deficit (VPD) was higher as the ECw increased from 0.5 to 6.0 dS m-1 (Fig. 4 D), ranging from 2.30 to 3.11 kPa, corresponding to an increase of 26% and evidencing that plants under salt stress are more vulnerable to a VPD, with a possible loss of water to the atmosphere.
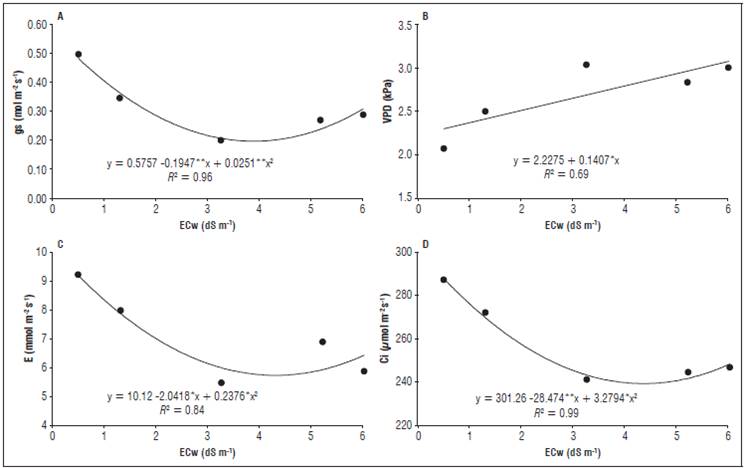
Figure 4 Stomatal conductance (gs), internal CO2 concentration (Ci), transpiration (E) and vapor pressure deficit (VPD) of the radish plants as a function of the different electrical conductivities of the irrigation water (ECw).
According to Parihar et al. (2015), the water restriction caused by high salt concentrations in the soil solution causes plants to close their stomata to increase water use efficiency and reduce the loss of water to the atmosphere, thereby reducing gs, E, Ci and A. Barboza and Teixeira Filho (2017), working with sugarcane, observed a higher gs early in the morning, when the VPD was low and the plants were hydrated, with the reverse seen in the late afternoon when the gs decreased and VPD increased.
As the gs increased, A, E, Ci also presented higher values (Fig. 5), corresponding to increases of 77.64, 58.97 and 82.24%, respectively. However, there was a reduction in the WUE, corresponding to a decrease of 78.07%. The variations ranged from 5.52 to 9.36 for E; 237.50 to 288.79 for Ci and 2.28 to 1.78 for WUE.
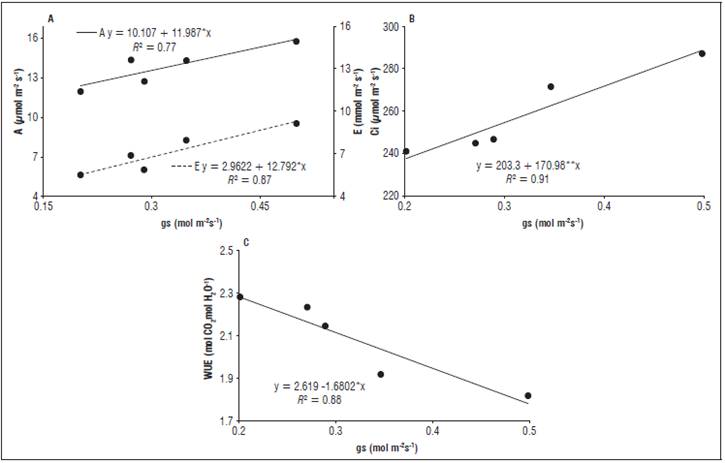
Figure 5 Net photosynthesis and transpiration (A), internal CO2 concentration (B) and water use efficiency (C) as a function of the stomatal conductance in the radish plants subjected to different electrical conductivities of the irrigation water.
According to these results, A, E, Ci and WUE had a strong relationship with the gs. The first three were directly proportional to the gs, and the latter were inversely proportional to the gs (Fig. 5). Under high gs conditions, Ci and A were favored by a greater assimilation of CO2, and E increased as a result of a greater number of open stomata, causing the plants to lose more water and consequently reducing WUE (Taiz et al.,2017).
As seen in the present study, Peloso et al. (2017) observed a directly proportional relationship between A and gs, where the gs increase provided higher A values in a coffee crop. Similarly, Barboza and Teixeira Filho (2017) reported that E was higher as sugarcane gs increased. Ferreira et al. (2011) also observed a directly proportional relationship between Ci and E and the gs and an inversely proportional relationship between USA and the gs in a soybean crop.
The reduction of gs is considered one of the main factors that restrict photosynthetic activity, decreasing the CO2 influx to the rubisco carboxylation sites within the chloroplasts and causing a decline in the photosynthetic rate. It also reduces E, providing less water loss and greater WUE (Tatagiba et al.,2015).
CONCLUSIONS
The electrical conductivity of the irrigation water (ECw) influenced the agronomic yield and gas exchange of the radish plants (Raphanus sativus L.) for gs, Ci, E and A, as a function of the gs. The foliar applications of ascorbic acid did not influence the agronomic yield or gaseous exchanges of the radish plants at the tested doses. The gs had a directly proportional relationship with A, E and Ci and an inversely proportional relationship with WUE















