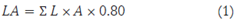INTRODUCTION
In Colombia, banana cultivation occupied 85,700 ha in 2016, of which 8,635 ha were in the municipalities of the banana production zone, Magdalena province, which generated 290,428 t, corresponding to 14.2% of domestic production. This production had an approx imate value of 2,093,985 million US dollars (Agronet, 2018). One of the tasks necessary for a better yield is adequate edaphic and foliar fertilization of the crop that is done properly. Phytohormones could be used as a complement to mineral nutrition, acting on the metabolic functions of plants (Albán, 2014). Among the main phytohormones, cytokinins stimulate plant growth and development (Mok, 2018). Within the group of cytokinins, agricultural applications in the field exist for kinetin, zeatin, zeatin riboside, trans-zeatin, c/s-zeatin, dihydrozeatin, 6-benzylaminopurine, and other natural and synthetic compounds (Sán chez and Mira, 2013). The physiological benefits of cytokinin applications to plants include stimulation of leaf growth, regulation of shoot and root develop ment, stimulation of respiration and photosynthesis, flower induction, and fruit growth (Jordán and Casaretto, 2006; Bar and Ori, 2014; Mok, 2018).
In Musaceae, according to the BBCH scale of develop ment, the vegetative meristem has a characteristic ar rangement of leaves with a spiral form, absence of lateral shoots and absence of internode growth (Nalina et al., 2006). The reproductive phase in this crop begins with changes occurring in the vegetative meri-stem, which undergoes metabolic shifts that trans form it into the floral bud (Sánchez and Mira, 2013; Landrein et al., 2018). In banana, flower induction depends on the number of emerged leaves (Rodríguez et al., 2012), which, in turn, relays on external fac tors and internal regulators, such as hormone activity (Chaurasia et al., 2017).
An important phase in the life cycle of banana plants is inflorescence development, where climatic condi tions including temperature, wind, and precipitation can delay flower differentiation and affect bunch formation (Galán et al., 2012). Air temperature influ ences the transpiration rate, as well as duration of life cycle and bunch weight in banana plants through the control that it exerts on leaf metabolism (Rob inson and Galán, 2012). Precipitation is one of the main climatic factors that determine banana crop development (Santos et al., 2018); plants can quickly adapt to a water deficit in soil, which would further severely reduce the rates of leaf emission and bunch development (Galán et al., 2012). The effects of edaphoclimatic conditions on flower induction and dif ferentiation in Musaceae could be explained, at least in part, by the hormonal interactions in plants (Rob inson and Galán, 2012).
According to previous studies, applications of plant growth regulators to Musaceae might shorten the juvenile phase of growth and accelerate flower dif ferentiation. Thus, the application of auxins at a rate 100 mg L-1 have stimulated floral differentiation in banana; however, treatments with naphthalene ace tic acid with doses exceeding 500 mg L-1 have caused fruit malformations (Lima et al., 2016). At the same time, applying gibberellins at doses of 500 to 1,000 mg L-1 has caused elongation of the pseudostem (Lima et al., 2016). Likewise, during vegetative growth, spray ing brassinolide at 3-6 g L-1 on banana (Musa sp. cv. Berangan) plantlets significantly has increased plant height, pseudostem diameter, leaf number, leaf area, and fresh and dry weight of plants (Zakaria et al., 2018). Applications of cytokinins, such as benzyladenine, at doses of 20 to 100 mg L-1 have increased the growth of corm in banana plants (Lahav and Gott-reich, 1984). According to Muriel (2012), cytokinins influenced cell division, retarded senescence, and in creased growth, fruit weight, and number of export able hands of banana.
In general, the effects of cytokinin applications on banana growth in the field are poorly studied. López (2014) evaluated applications of cytokinins, compar ing two fertilizer alternatives injected into the pseud ostem of Musa sp. These treatments had no positive effect on the plant growth; one of the treatments contained kinetin at 1 g L-1 (López, 2014). In the veg etative propagation of Musa sp. with 6-benzylamino-purine (BAP) and indoleacetic acid (IAA), the best treatment was 30 mg L-1 BAP, which positively in fluenced shoot formation in each studied variety, fol lowed by the concentration 30 mg L-1 BAP + 10 mg L-1 IAA (Canchignia et al., 2008). Albán (2014) found positive effects from cytokinin applications on leaf emission, increasing the pseudostem diameter in cv. Grand Naine; in addition, foliar applications of algae extracts rich in cytokinins were a source of hormones and essential carbohydrates, which resulted in bet ter yield and harvest quality (Albán, 2014). Aspiazu (2014) compared the applications of various hor mones, concluding that gibberellins (20 mg L-1) and brassinosteroids (2 mg L-1) positively influenced plant growth and increased leaf length in banana. Howev er, when 20 mg L-1 gibberellin, 20 mg L-1 cytokinin, and 2 mg L-1 brassinosteroids were jointly applied, no significant increases were found in the pseudostem diameter, number/weight of roots or leaf width (Aspiazu, 2014). Langford et al. (2017) studied the macropropagation of banana plants in a nursery with BAP at two concentrations: 10-2 M and 5 x 10-3 M, with the following treatments: immersion of corms in BAP solutions for 30 min, immersion of corms in coconut water for 30 min, and placing the corms in a substrate of rice husk. The treatments with BAP and coconut water (a natural source of cytokinins) induced sprouting in the corms and a loss of the api cal dominance (Langford et al., 2017).
The use of phytohormones gibberellins, brassino-steroids, and cytokinins, individually or mixed, is mainly studied in the propagation of Musa sp., such as in the asexual multiplication of corms planted in the field (Canchignia et al., 2008). However, no reports were found in the literature on the use of trans-zeatin riboside as a possible factor increasing vegetative growth in banana. The objective of the present research was to evaluate the effect of ap plications of trans-zeatin riboside (cytokinin) on the vegetative growth of banana cv. Williams in the Mag dalena Province of Colombia.
MATERIALS AND METHODS
Experiment locations
This field study was carried out on two farms: El Polo located at 10°53'39.905" N and 74°11'58.214" W and La Paz 1 located at 10o53'37.17'' N and 74o11'54.623" W, both at an altitude of 20 m a.s.l. in the town El Mamey (Colombia), and characterized with a tropical dry climate according to Holdridge (Aguirre, 2012). This study was conducted between October 2016 and January 2017. The duration of the field experiment was 13 weeks.
The climatic data in the field were obtained from the meteorological station El Enano of the Colombian meteorological institute INAT (El Mamey). The aver age air temperature was 26.8oC, with monthly pre cipitation of 12.8 mm and an average wind speed of 14.54 km h-1. In general, the climatic conditions were typical for areas of commercial banana production on the Atlantic coast of Colombia, which are adequate for the development of banana plants (Sánchez and Mira, 2013). The two farms, where the plants were established, differed in physical and chemical char acteristics of the soil. The soil type on both was Inceptisoles (Soil Survey Staff, 2010). The marked differences between the two locations were in soil texture (La Paz 1: Loamy, El Polo: Sandy Loam); pH (La Paz 1: 5.8, El Polo: 7.7), C/N ratio (La Paz 1: 11, El Polo: 12.2), and cation exchange capacity (La Paz 1: 16.9, El Polo: 9.4 meq 100 g-1) of the soil arable layer.
Field crop management
Banana (Musa AAA Simmonds) cv. Williams plants, propagated in vitro, were employed. Prior to trans plant to the field, the plants were hardened in a shade house, in which water and mineral nutrients shade house, in which water and mineral nutrients were supplied as edaphic and foliar applications for 6 weeks.
The plants were established in the field at week zero with 4 leaves and 25 cm maximum height at the time of transplant; the planting density was 2.40 m between the plants and 2.40 m between the rows. In the sowing sites, 60 g per plant of Rafos® (Yara, Colombia) edaphic fertilizer were applied. The plant management practices employed by Torres (2016) for conventional banana produced for export in Colom bia were used.
The irrigation with sprinkling was done between weeks 0 and 13 according to the needs of the crop. Between weeks 0 and 6 after planting, 6 mm ha-1 of irrigation water were applied daily, divided in two ir rigation periods. Starting from week 7 after planting, 5 mm ha-1 water were applied per day; for this, a sub-foliar spray system was used, with 2014 Senninger® sprinklers spaced at 10 m by 10 m.
Every 15 d during weeks 0-12, edaphic fertilizers were applied at a rate of 60-120 g/plant. On the La Paz 1 farm, Rafos® was applied at 60 g/plant (week 0), Ammonium sulfate at 90 g/plant (week 2), Ami das® (Yara, Colombia) at 90 g/plant (week 4), Cal cium Nitrate at 90 g/plant (week 6), and Aboteck® (Yara, Colombia) at 90 g/plant (weeks 8 and 10) and 120 g/plant (week 12). On the El Polo farm, the same products were used but the doses were different: 60 g/plant (week 2), 60 g/plant (week 4), and 90 g/plant (week 12). Every 15 d starting from week 1, foliar fertilizers (Wuxal®, Bayer, Colombia) were applied were supplied as edaphic and foliar applications for using a 20 L mechanical back pump, prepared with 6 weeks. 100 cm3 of molasse water.
The mechanical weed control (Quintero-Pertúz and Carbonó-Delahoz, 2015) was done every 15 d. Every week, the lower leaves affected by Black Sigatoka (Mycosphaerella fijiensis) were eliminated until week 5. Starting from week 6, only the fraction of the leaf af fected by Black Sigatoka was removed. The products applied to control Black Sigatoka were Sico-Dithane® (3rd week), Siganex-Dithane® (5th week), Opus-Dithane® (8th week), and Voley-Dithane® (12th week). The suckers were cut off the plants every 6th week.
Applications of trans-zeatin riboside
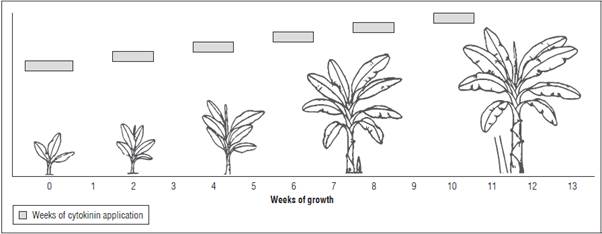
The hormone trans-zeatin riboside (t-ZR), 95% pu rity, was obtained from Sigma® laboratories (Sigma-Aldrich, St. Louis, MO), stored according to the manufacturer recommendations at -20oC, and dilut ed in type 1 water before the applications to reach the dose required in each treatment. Four treatments were tested on the plants via foliar spraying: water (control, 0 mg L-1 trans-zeatin riboside), 0.05 mg L-1, 0.25 mg L-1, or 0.45 mg L-1 of trans-zeatin riboside. Applications were made every 15 d (Fig. 1) using a manual back pump with a 21 pound pressure regu lator and yellow nozzle (GEF-REPCar, 2011), spray ing the plant leaves and pseudostem. The treatments started at planting in the field and continued until completing 6 applications of the hormone in each plant. The applications of cytokinin ended at week 10 after transplant (Fig. 1).
Data collection in the field
The experiment had a bi-factorial randomized block design with six replicates (one plant per replicate) on the La Paz 1 and El Polo farms. A total of 360 plants were planted; in each location, 20 plots were ar ranged, of which, 15 plots had 6 plants and the other five plots had 18 plants.
From week 1 and up to week 13, in each research plot, biometric data were collected weekly on each plant: leaf length (in each leaf), leaf width (in each leaf), pseudostem diameter, and plant height. From the ground to the bifurcation point of the pseudostem, the height of the plant (cm) denominated as "height in V" was measured with a tape measure. The diam eter of the pseudostem was measured with a caliper, 5 cm from the ground. The area of each leaf was cal culated with the following equation (1) (Martínez et al., 2015):
where LA is leaf area (cm2), L is length of the leaf blade from the apex of the leaf to its base (cm), and A is width of the leaf blade in the middle part (cm).
Statistical analysis
The statistical analysis used one-way analysis of vari ance, with a Tukey test at a confidence level of 95%. For all cases, the assumption of normality of the Shapiro-Wilk residuals and Bartlett's variance equality was tested. The area under the curve was calculated using the AUDPS function of the Agricolae® package (Simko and Piepho, 2012). To identify statistically significant differences between the means of the vari ables, the Student t test (P<0.05) was used.
RESULTS AND DISCUSSION
Plant height
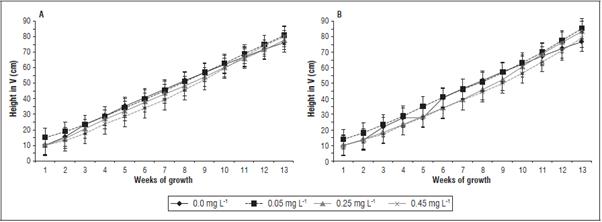
On the farm La Paz 1, treatments with 0 and 0.05 mg L-1 trans-zeatin riboside resulted in a significantly larger plant height in V (height of the plant from the ground level up to point of the pseudostem bifurca tion), as compared to the treatments with 0.25 and 0.45 mg L-1 cytokinin (Fig. 2A). The tallest plants were obtained in the treatment with 0.05 mg L-1 cytokinin, with a 46.3 cm average height (average value of mea surements between weeks 1 and 13) (Fig. 2A). On El Polo, the four treatments had different heights, with the tallest plants, 46.9 cm average height, obtained after applications of 0.05 mg L-1 cytokinin (Fig. 2B). Apparently, this indicated that trans-zeatin riboside, at a rate of 0.05 mg L-1, stimulated cell growth (cell division or/and cell elongation), which resulted in an increased height. These results are consistent with the research of Ortiz et al. (2013), in which cytokinin applications generated a faster growth in Musa sp. In banana, the pseudostem diameter and height-to-circumference ratio (HCR) for tall cultivars as well as HCR for medium-height cultivars are known as good predictors of inflorescence emergence since these variables have exhibited linear or quadratic relationships with the number of days from planting to inflorescence emergence (Vinson et al., 2018).
Pseudostem diameter
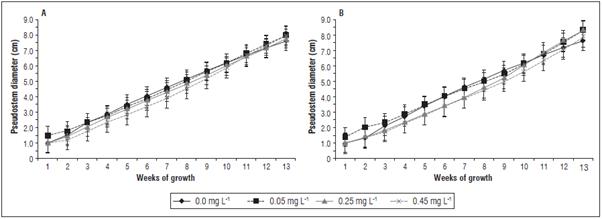
The diameter of the pseudostem was assessed as a variable of plant development; all treatments in creased the pseudostem diameter on the farms La Paz 1 and El Polo over time. However, the plants grown on La Paz 1 (Fig. 3A) differed in the confidence inter vals for the treatments 0 and 0.05 mg L-1 , as com pared to the treatments 0.25 and 0.45 mg L-1; the treatment 0.05 mg L-1 yielded the highest average pseudostem diameter, 4.6 cm (average value of mea surements between 1 and 13 weeks). On the El Polo farm, the plants treated with 0.05 mg L-1 cytokinin had the largest average pseudostem diameter, 4.6 cm (Fig. 3B). These results indicate that the best treat ment in both locations was 0.05 mg L-1 trans-zeatin riboside, which was the lowest dose. The increase in pseudostem diameter could indicate a higher cellular activity, which implies increases in dry mass, higher demand for nutrients, and a higher meristematic ac tivity (Sánchez and Mira, 2013). In Musa sp., a large pseudostem diameter correlates with a higher plant resistance to breakage by wind and reflects a plant's ability to sustain the bunch (Gonçalves et al., 2018), as well as correlates with a higher storage capacity in the pseudostem for water and carbohydrates (Sán chez and Mira, 2013; Shivashankar et al., 2016).
Leaf growth variables
The leaf length did not present significant differenc es between the treatments (Fig. 4), while on La Paz 1, the leaves tended to be the longest with the 0.25 mg L-1 cytokinin application, with an average length value of 58.5 cm (average value of measurements be tween 1 and 13 weeks) and, on El Polo, the best treat ment was 0.45 mg L-1, with a length value of 63.1 cm (Fig. 4 A and B).
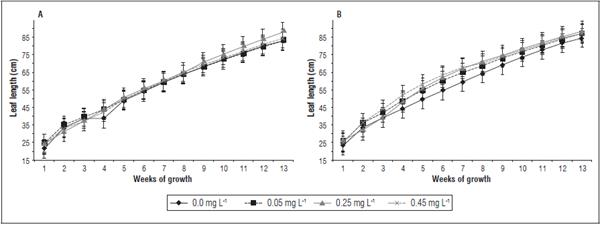
Figure 4 Leaf length of banana plants cv. Williams sprayed with different doses of trans-zeatin riboside during vegetative growth on the farms La Paz 1 (A) and El Polo (B). The results are presented as mean ± standard error.
At the same time, the leaf width significantly differed between the treatments (Fig. 5). On La Paz 1, the widest leaves were obtained in the treatment with 0.25 mg L-1 cytokinin, where the average leaf width reached 28.3 cm (average value of the measurements between 1 and 13 weeks) (Fig. 5A). On El Polo, the largest leaf width was recorded in the 0.05 mg L-1 treatment, with a 29.9 cm average value (Fig. 5B). As a result, the plants on the El Polo farm presented the widest leaves. This effect from the cytokinins was consistent with the results of Bar and Ori (2014), who indicated that leaf blade development depends on light and cytokinins for maintaining balance with auxins and stimulation of meristem activity. Cytokinins are known to stimulate cell division and cell expansion in leaves (Mok, 2018). In a review carried out by Landrein et al. (2018) on Arabidopsis, expres sion of the CYCD3 gene is required for development of new leaves until reaching the leaf size typical of this species.
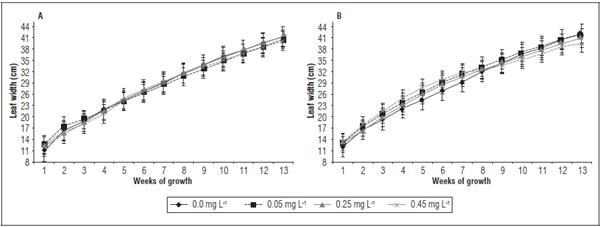
Figure 5 Leaf width of banana plants cv. Williams sprayed with different doses of trans-zeatin riboside during vegetative growth on the farms La Paz 1 (A) and El Polo (B). The results are presented as mean ± standard error.
The leaf area reflected a relationship between leaf length and width, presenting no significant differ ences between the locations or between the treat ments on La Paz 1 and El Polo. The highest average leaf area on La Paz 1 was reached with 0.25 mg L-1 cytokinin, 1473 cm2 (average value of measurements between weeks 1 and 13) and, on the El Polo farm, the 0.05 mg L-1 cytokinin treatment resulted in the highest average leaf area, 1616 cm2 (Fig. 6A and B). It can be speculated that the phytohormone spray ing on the leaves/pseudostem affected expression of the LOG genes in the apical meristem region of the stem, where the stem cells reside. In Arabidopsis, the LOG4 gene is expressed in the L1 layer of the vegeta tive meristem; LOG genes encode enzymes that con vert inactive cytokinin ribosides into active forms, providing a localized source of active cytokinin in the vegetative meristem (Landrein et al., 2018). Cytokinins could increase leaf size because of the high rate of cell expansion, yielding a higher shoot biomass (Skalák et al., 2019) and delayed leaf senescence (Gan, 2014). In banana, direct relationships between leaf area and yield were previously established (Robinson and Galán, 2012); these data are important since the growth and formation of banana bunches depends on the number and physiological activity of functional leaves (Rodríguez et al., 2012).
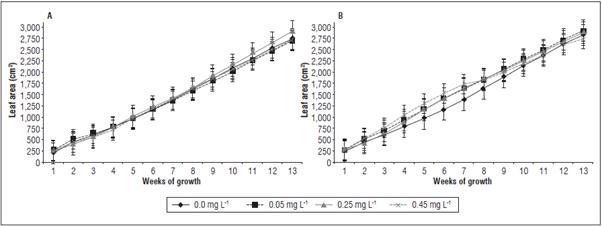
Figure 6 Leaf area of banana plants cv. Williams sprayed with different doses of trans-zeatin riboside during vegetative growth on the farms La Paz 1 (A) and El Polo (B). The results are presented as mean ± standard error.
The results of the present study indicated that the application of trans-zeatin riboside positively influ enced the development of banana plants without a reduction in growth or presenting deformations in plant morphology, such as the ones reported by Albán (2014). In our research, the treatment that gen erated, on average, the highest leaf expansion was 0.05 mg L-1 trans-zeatin riboside, as compared with the other evaluated treatments. These findings agree with the data reported for applications of other rates/ formulae of cytokinins, which tended to increase the growth rate of plants, resulting in increases in height, pseudostem diameter, leaf length and leaf width in Musa sp. plants (Albán, 2014; Aremu et al., 2014; Aspiazu, 2014).
The practical importance of faster vegetative growth in Musaceae at the commercial level includes a reduc tion of the juvenile phase of growth, which favors a change from vegetative to reproductive growth, accelerates flower differentiation, and reduces the number of weeks required for flowering and initia tion of bunch formation. The applications of trans-zeatin riboside on Musa sp. plants during vegetative development could be explored for possibly counter acting the effect of stress caused by biotic and abiotic factors (Scháfer et al., 2015; Miller et al., 2017), which might further increase production levels on commer cial plantations.
CONCLUSIONS
The growth variables height in V, leaf width, leaf area, and pseudostem diameter presented statisti cally significant differences, depending on the level of trans-zeatin riboside sprayed on the banana plants cv. Williams during 10 weeks of growth in the field. The low doses of trans-zeatin riboside, such as 0.05 mg L-1, generated the best results for plant height at V, pseu dostem diameter, leaf width and leaf area