INTRODUCTION
Lippia alba (Mill.) N.E.Br. (lemongrass, Verbenaceae) is a plant of medicinal interest, and tea made from its leaves has a soothing effect (Hohlenwerger et al., 2017). It can also be used as a natural preservative (Machado et al., 2011). The essential oil from its leaves has shown an antifungal activity against Aspergillus, Fusarium, Pénicillium and Trichoderma (Glamoclija et al., 2011). There are few studies on cultivation prac tices that must be established during the production of seedlings propagated with stem cuttings.
The abiotic factors that may influence the vegeta tive propagation of seedlings include light availabil ity in the cultivation environment, which may affect the morphophysiological responses of plants since it acts directly in the process of photochemical and biochemical reactions of photosynthesis (Leal et al., 2015).
However, light intensity may cause damage to the photosynthetic apparatus (Díez et al., 2017; Taiz et al., 2017) by changing the carboxylation speed of Ribulose 1.5 bisphosphate carboxylase/oxygenase, the assimilation of CO2 and, consequently, the pro duction of photoassimilates for seedling formation. The plants have foliar functional strategies based on chlorophyll-a fluorescence in photosystem II (PS II), enabling the efficient use of light (Jaimez et al., 2018; Jiménez-Suancha et al., 2015). Therefore, these photochemical parameters may be used in assessing the integrity of photosynthetic machinery under eco-physiological disorders as a result of light stress.
In addition to light, another limiting factor for plants is soil in tropical regions, such as soil in Cerrado, which is highly weathered, has high levels of iron and aluminum oxides, and has high acidity (Souza et al., 2013; Islas-Espinoza et al., 2014), limiting the avail ability of some nutrients, particularly phosphorus (P). Greater attention is required for the low avail ability in soil in the tropics because of the greater fix ation in soil (Quesada et al., 2011; Gérard et al., 2016).
Generally, P-deficient plants may suffer damage in diffusive and non-diffusive processes of photosynthetic metabolic pathways through a reduction in consumption and regeneration of Rubisco and pro duction of ATP and NADPH (Andrade et al., 2018), which may cause instability in the photochemical process in PS II. Thus, phosphate fertilization is an important agronomic practice since it may assist in photochemical stability (Carstensen et al., 2018) and biomass increases in plants (Kuwahara et al., 2016).
We tested the hypothesis that P may contribute to the production and quality of seedlings, mitigate damage and increase photochemical reactions in PS II under light stress. This study associated the mineral nutrition and the ecophysiology of the plants in order to assess the photochemical and vegetative response and quality of L. alba seedlings produced with stem cuttings under different light availability and phos phate fertilization.
MATERIAL AND METHODS
Collection of plant material and preparation of cuttings
This species was identified and a voucher was de posited at the Herbarium DDMS of the Federal Uni versity of Grande Dourados (UFGD), under number 5226. The collection of plant material was performed from plant matrices in the Horto de Plantas Medici nais (22°11'43.7"S and 54°56'08.5"W 452 m a.s.l.) of UFGD, in good phytosanitary conditions. The cut tings were removed from the median portion of the branches, standardized as 20 cm in length, mean di ameter of 2.45 mm, and a pair of leaves at the apex; 1/3 of the cuttings was buried in the substrate.
Studied Factors and experiment design
This experiment was performed from October to December of 2017 in the Faculty Agrarian Science (22°11'43.7"S e 54°56'08.5"W 452 m a.s.l.) of UFGD in Dourados, Mato Grosso do Sul, Brazil. The climate of the region is classified as Am (Alvares et al., 2013) with an annual average rainfall over 1,500 mm. The factors consisted of two light conditions (full sun and 50% shading) and four levels of phosphorus in the form of simple superphosphate - 18% of P2O5 (0, 150, 300 and 450 mg kg-1). The treatments were displayed in a 2x4 factorial scheme, in randomized blocks, with four replicates. The experiment unit consisted of five 500 mL plastic containers, with one cutting each.
Shading was simulated using polypropylene black screen with 50% retention of light. The soil was clas sified as Dystroferric Red Latosol (Santos et al., 2018), clay texture, with the following chemical attributes: pH CaCl2 = 4.76; P= 0.5 mg dm-3; Ca = 1.04 cmolc dm-3; K = 0.06 cmolc dm-3; Mg = 0.12 cmolc dm-3; Al = 1.2 cmolc dm-3; H+Al = 7.71 cmolc dm-3; sum of bases = 1.22 cmolc dm-3; cation exchange capacity = 8.93 cmolc dm-3 and base saturation = 13.68. No soil correction was performed. Base fertilization was per formed in coverage with ammonium sulphate (20% N) and potassium chloride (60% K2O). The cultivation practices consisted of daily irrigation at 70% of the substrate's field capacity.
Chlorophyll-a fluorescence
At 30 and 60 days after burial (DAB) of cutting, the emission of chlorophyll-a fluorescence was assessed by subjecting the leaves to darkness for 30 min, using leaf clips, between 8:00 and 10:00 am. The initial (F0) and maximum (F m ) chlorophyll-a fluorescence and photochemical efficiency of photosystem II (Fv /Fm) were measured under flash of 1,500 µmol m-2 s-1 with a portable fluorometer (OS-30P; Opti-Sciences Chlo rophyll Fluorometer, Hudson, NY). The variables flu orescence (Fv = Fm- F0), efficiency of absorbed energy conversion (Fv /F0), non-photochemical maximum yield (F0 /F v ) and electron transport rate (ETR) were estimated (Baker, 2008).
Quantification of photosynthetic pigments
At 60 DAB, fully expanded leaves were collected, with 1 g macerated in 8 mL of acetone (80%). After wards, the samples were centrifuged for 10 min at 3000 rpm, and the absorbance reading was taken at the wavelengths of 470, 645 and 663 nm using a spectrophotometer. The contents of chlorophylls a, b, to tal (a + b) and carotenoids were estimated (Arnon, 1949; Lichtenthaler and Buschmann, 2001).
Growth indicators
After 65 DAB, the survival, length of the aerial part (distance from the collet to the inflection of the high est leaf), collar diameter - CD (± 1.0 cm above the substrate level), leaf thickness, and number of buds and leaves were recorded. Seedlings were removed from the containers, washed and assessed for length of the largest root and rooted cuttings (emission of adventitious root of at least 5.0 cm). The leaf area was also assessed (LA) using an area integrator (area meter LI-COR 3100 C; Licor, Lincoln, NE).
Biomass, physiological and quality indices
Fresh material from the aerial and root parts were submitted to forced air circulation in an oven at 60±5oC to obtain the dry mass. Using the data for the LA and leaves and total dry biomass (LDM and TDM, respectively), the physiological indices of leaf area ratio (LAR = LA/LDM), specific leaf area and mass (SLA = LA/TDM and SFM = LDM/TDM, re spectively) were calculated (Benincasa, 2003). From the data for total dry biomass, height/diameter ratio (RHD) and aerial part/root ratio (APRR), the Dick son quality index (DQI) was estimated (Dickson et al., 1960).
Statistical analysis
The rooting and survival data were transformed into arcsine of √x + 0.5 and subjected to normal distri bution with the Shapiro-Wilk test for normalization. The data for chlorophyll-a fluorescence were assessed in plots subdivided in time. All data were subjected to analysis of variance (ANOVA), and, when significant according to the F test, the averages were compared with Student's t test for the luminosity and evalua tion periods, along with regression analysis for phos phorus (P<0.05), using SISVAR.
Additional multivariate analysis of principal compo nents was carried out with variance and co-variance arrays. A cluster analysis was also performed using the complete linkage method to describe the similar ity between the factors, and the grouping was per formed with the classical method using Euclidean distances.
RESULTS
The greatest seedling survival and collet diameter were observed under full sun (Fig. 1A and B). The greatest height/diameter ratio (RHD) occurred in the shaded seedlings (Fig. 1C). The leaf number (LN) was influenced by the factors independently, with higher amounts under the shaded environment (Fig. 1D), without adjustment to phosphate fertilization (Tab. 1).
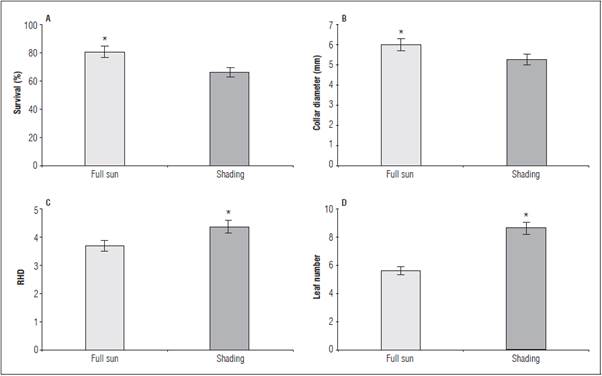
Figure 1 Survival (A), collar diameter (B), height/diameter ratio - RHD (C) and leaf number - LN (D) in L. alba seedlings pro duced under light ambience. * significant difference according to the Student's t test (P≤0.05).
Table 1 Leaf and budding numbers, chlorophyll b, variable fluorescence, leaf fresh and dry mass in L. alba seedlings produced with different phosphorus doses.
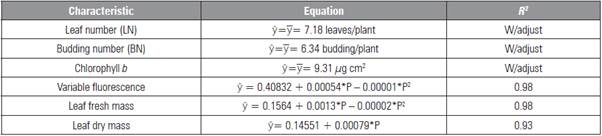
* significant difference (P≤0.05).
The characteristics height, rooting, length of the larg est root, stem fresh and dry mass, root fresh mass and total mass were not influenced by the factors (P>0.05), with averages of 22.4 cm, 76%, 12.0 cm, 2.22, 1.04, 0.96 and 6.30 g/plant, respectively. The number of buds, chlorophyll b and fresh and dry mass of the leaves were influenced only by phosphorus, in which the data for LN, NB and chlorophyll b had no adjustment to the mathematical models (Tab. 1) be cause of the low value of the determination coefficient (R 2 ).
Chlorophyll a and total showed the same trend in the shaded environment, reducing the levels with increasing doses, with maximum levels (17.62 and 28.19 µg cm2) and minimum levels (7.74 and 10.21 µg cm2) without and with (450 mg kg-1) P, respectively (Tab. 2). On the other hand, under full sun, chloro phyll a and carotenoids had higher contents (9.96 and 1.84 µg cm2, respectively) with the addition of 450 mg kg-1 of P.
Table 2 Chlorophyll a, total chlorophyll, carotenoids, pho tochemical efficiency of the photosystem II (F v /F m ), maximum non-photochemical yield (F0/Fv) and efficiency of absorbed energy conversion (Fv/F 0 ) in L. alba seedlings produced with phosphorus under light environment.
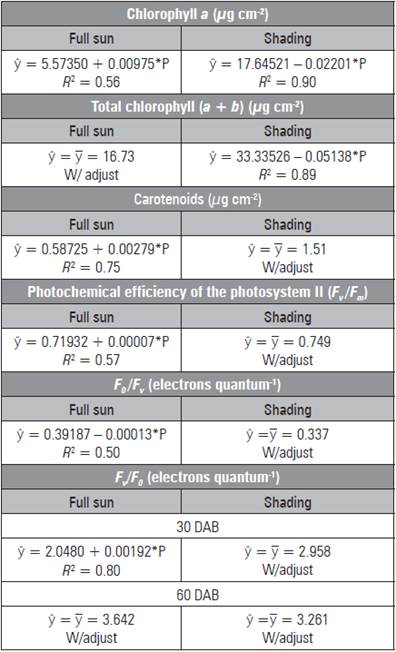
* significant difference (P≤0.05).
Under the shaded environment, the data for F v /F m and F 0 /F v had no adjustment to the mathematical models (Tab. 2). Under full sun, the highest ratio was 0.750 and 0.338 electrons quantum-1, under 450 mg kg-1 of P, respectively. The higher F v /F m values were observed at 60 DAB in full sun as a result of the low est non-photochemical yield (F0/Fv) (Tab. 3). The greatest F 0 occurred in the leaves of shaded seedlings (0.162 electron quantum-1) (Fig. 2A) and at 30 DAB (0.187 electrons quantum-1) (Fig. 2B).
Table 3 Photochemical efficiency of the photosystem II (F v /F m ) and maximum non-photochemical yield (F 0 /F v ) in L. alba seed lings produced with phosphorus under light environment, at 30 and 60 days after burial (DAB).
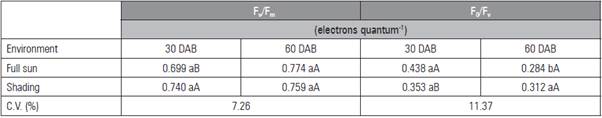
Means followed by the same lower case letter in the rows, for day after burial, and upper case in the columns, for light environment, do not differ according to the Student's t test (P≤0.05).
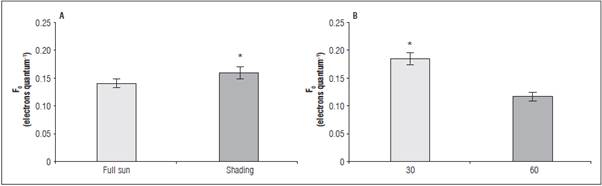
Figure 2 Initial fluorescence - F 0 in leaves of L. alba seedlings produced under phosphorus rates and light environment (a), at 30 and 60 days after burial (b). *significant difference according to the Student's t test (P<0.05).
The maximum F v 0.415 electron quantum-1 was found with the addition of 27 mg kg-1 of P (Tab. 1). The shaded seedlings had higher F v and F m values at 30 DAB, with a reduction of values at 60 DAB in both environments (Tab. 4). The ETR had lower values at 30 DAB (Tab. 4). The greatest ETR occurred in the leaves of seedlings cultivated under full sun at 60 DAB as a result of the higher incident light.
Table 4 Maximum (Fm) and variable (F v ) fluorescence and electron transport rate (ETR) in leaves of L. alba seedlings produced with phosphorus under light environment at 30 and 60 days after burial (DAB).
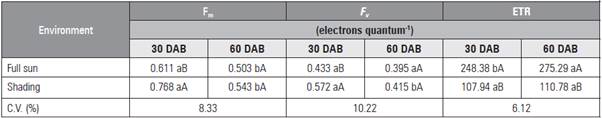
Means followed by different lower case letters in a row, for day after burial, and upper case letters in the columns, for light environment, indicate significant diffe rences according to the Student's t test (P≤0.05).
In the shaded environment, the largest LA was 16.43 cm2 under 450 mg kg-1 of P. Under full sun, the maxi mum leaf area (11.85 cm2) occurred in seedlings cul tivated with 228.31 mg kg-1 of P (Tab. 5). The leaf thickness data were not adjusted, and the sun leaves had higher values. The data for root dry mass and APRR of the seedlings under full sun were not adjust ed to the mathematical models, but, under the shad ing, the higher mean values were 0.462 g/plant and 0.44 with 450 mg kg-1 of P. The fresh and dry biomass of the leaves were influenced only by phosphorus, with higher values (0.177 and 0.0501 g/plant) with the addition of 32.5 and 450 mg kg-1 of P, respectively (Tab. 1).
Table 5 Leaf area, thickness, root dry mass and aerial part/ root ratio in L. alba seedlings produced with phos phorus under light environment.
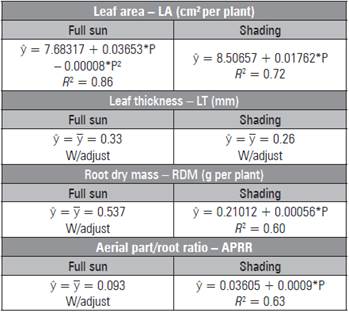
* significant regression (P≤0.05)
The greatest LAR (2.027 cm2 g-1) and SLM (0.0458 g cm-2) occurred in the shaded seedlings (Fig. 3A and B) as a result of the greatest leaf thickness (Tab. 5). The greatest SLA was 48.54 cm2 g-1 in the seedlings pro duced in the shaded environment (Fig. 3C). For the DQI, the greatest value (1.70) was observed in the seedlings cultivated under full sun (Fig. 3D), regard less of phosphorus.
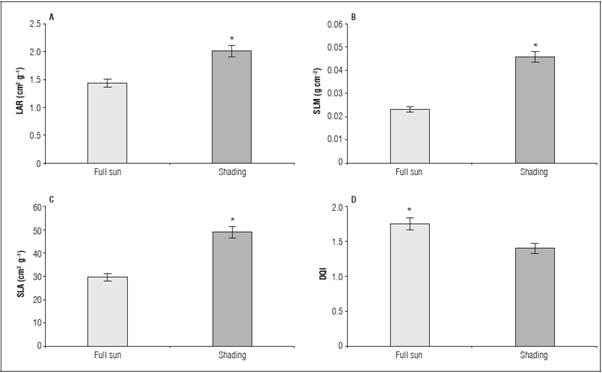
Figure 3 Leaf area ratio - LAR (A), specific leaf mass - SLM (B), specific leaf area - SLA (C) and Dickson quality index - DQI (D) in L. alba seedlings under light environment at 65 days after burial. "significant difference according to the Student's t test (P≤0.05).
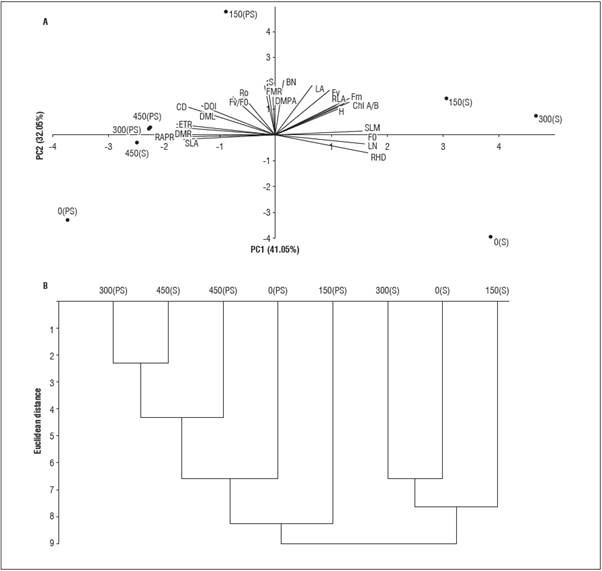
The principal components analysis explained 74% of the variability, in which PC1 and PC2 contributed 41.05% and 32.05%, respectively, of the remaining variance of the characteristics in the L. alba seed lings (Fig. 4A). In the cluster analysis, two groups were formed for the P dose (G1 and G2), with six subgroups (Fig. 4B). The subgroups with lower dis tances between P dose and light environment (S -shading and PS - full sun) were 300 PS and 450 S (2.31), followed by 450 S and 450 PS (4.61) and 300 S and 0 S (6.65).
DISCUSSION
The greatest survival was associated with the amount of photoassimilates as a function of greater incident light. Seedlings under excessive light lose more water through transpiration because of an increased temperature, with the greater diameter allowing a greater ability to transport water and photoassimilates to other organs in order to maintain turgidity (Taiz et al., 2017). The greatest RHD occurred in the shaded seedlings, but the values did not indicate that there was etiolation in the seedlings under limited light.
Generally, plants under shading tend to expand the number of leaf limbs, enabling greater light inter ception (Oliveira et al., 2017) and maximizing pho tosynthesis. These characteristics are important in the vegetative propagation of the species since the greater the amount of buds, the greater the forma tion of leaves, contributing to photoassimilate distri bution to developing organs.
The reduction of pigments, except carotenoids, in leaves under full sun can be explained by photoin hibition of chlorophylls as a result of excessive light (Fu et al., 2012). However, shaded leaves tend to increase the content of chlorophyll and, thereby, maximize the photosynthetic capacity (Díez et al., 2017). Simi lar results were observed by Soares et al. (2017) when assessing young Tamarindus indica L. plants under shading, which presented higher concentrations of chlorophylls.
An increase in chlorophyll content is desirable be cause it is responsible for capturing light photons and transmitting energy to reaction centers (Monteiro et al., 2018), and carotenoids are responsible for chlo rophyll photoprotection and reduction of membrane harm (Taiz et al., 2017), maximizing the photosynthetic capacity. Under full sun cultivation, phosphate fertilization can be a potential mitigation strategy for ecophysiological disorders from light stress in the synthesis of chlorophylls.
P, in the form of Pi, is responsible for the control of enzymatic reactions and metabolic regulation in the cytosol and chloroplast (Hawkesford et al., 2012). Higher photochemical indicators, especially the Fv/Fm ratio, with phosphorus result because P favors the speed of ATP synthesis, contributing to the export of protons to the chloroplast stroma and thylakoid lumen (Carstensen et al., 2018), favoring the main tenance of electron mobility in PS II. Increasing F 0 /F v can be a photoprotective mechanism of the photosynthetic apparatus for excessive incident radiation (Blind et al., 2018), i.e. a greater use of electrons pro duced as a result of higher efficiency in absorbed en ergy conversion (F v /F 0 ), regardless of the shading level during this period (60 DAB) (Tab. 2).
The higher F 0 at 30 DAB is desirable in order to mitigate damage in the photosynthetic apparatus (Fig. 2B). Fu et al. (2012) described a higher F 0 in Lactuca sativa L. plants cultivated under 100 µmol m-2s-1 (low irradiance) at the initial stage of the experiment, stat ing that this mechanism can mitigate an increase in reactive oxygen and D1 protein degradation. As for phosphorus in F v , P is involved in phosphorylation reactions and pyrophosphate release, acting in the activation of catalytic enzymes, forming ATP (Hawkesford et al., 2012, Xing and Wu 2014; An drade et al., 2018) and favoring photochemical sta bility because F v is related to the ability to transfer electrons.
The results for F m and ETR is important in the photochemical process since larger values favor the flow of chlorophyll molecules between acceptors in photosystems (Farias et al., 2016). The higher photo chemical efficiency in PS II effectively contributes to maintaining the integrity of the photosynthetic ap paratus and increasing vegetative characteristics.
Plants exposed to low luminosity enhance the ex pansion of leaves (Gondim et al., 2018) as a strategy for use of light. Increasing APRR and RDM may be associated with the fact that under high irradiance, substrate and leaves tend to lose more water through evapotranspiration and leaf transpiration. Thus, an increase in these characteristics under full sun promotes water absorption for the maintenance of metabolic processes and nutrient transport (Sarto et al., 2018).
The responses of plants to abiotic variants can change according to the species. Oliveira et al. (2017), when assessing the physiological and produc tive aspects of Origanum vulgare L. plants, found great er root biomass under shaded cultivation. On the other hand, there was greater biomass allocation in Physalis minima L. seedlings under full sun (Silva et al. 2016), similar to L. alba seedlings (Fig. 3).
P plays an important role in plant biomass allocation and is a structural component of nucleic acids, phospholipids and ATP formation, favoring primary me tabolism reactions and constituting ~ 0.2% of plant mass (Kuwahara et al., 2016). For seedling production in Dalbergia nigra Benth., it was found that the addi tion of 500 mg kg-1 of P favored an increase in biomass (Carlos et al., 2018), similar to the L. alba seedlings in this study (Tab. 1).
LAR and SLM show greater biomass allocation through leaf area with greater thickness (Oliveira et al., 2016). These authors found that Melissa officinalis L. plants, at 120 d after transplanting, showed a greater SLM when cultivated under full sun. For LAR, Ribeiro et al. (2018) found higher values in Pogostemon cablin cultivated under shaded conditions. The increase in SLA under this condition indicated the adaptive ability of leaf tissues in optimizing light capture (Guzmán et al., 2016; Liu et al., 2016) because it promotes CO2 assimilation and stomatal control (Gommers et al., 2013). However, leaves with a small er thickness are less heavy. Similar results were ob served in Enterolobium contortisiliquum (Vell.) Morong (Souza et al., 2017) and Colocasia esculenta L. Schott (Gondim et al., 2018). Both authors described higher values in plants under artificial shading.
The DQI demonstrated that this species has surviv ability and stability capacity when exposed to high irradiance, an important fact since the initial cultiva tion under this condition reduced the acclimatization period (rustification) of the seedlings, i.e. these will be less susceptible to weather under field conditions, such as excessive sunlight. The use of DQI has been constant when assessing seedling quality since it is an easy implementation analysis, performed by cal culating the morphometric stability level, distribu tion and biomass allocation.
In PC 1, the characteristics that showed positive scores in descending order were RHD (0.271), LN (0.263), F 0 and SFM (0.255), and the characteristics with negative scores were APRR (-0.292), ETR and RDM (-0.288) and SLA (-0.270), which were the most similar. In PC 2, the components that most contrib uted with positive factorial scores were the number of buds (0.352), survival (0.330), leaf area (0.319) and F v (0.289), with no negative absolute scores > 0.30. The cluster analysis consisted of sample classification in order to verify the similarity within the groups and between-group heterogeneity, considering all evalu ated characteristics (Araújo et al., 2013). Thus, there was greater heterogeneity between the luminous en vironments, with G1 comprising the association of P with shading, except for 450 S and G2 under full sun.
CONCLUSION
The Lippia alba seedlings responded positively to the phosphate fertilization when vegetative propagation was used with stem cuttings. The association of 450 mg of phosphorus with the cultivation under full sun contributed substantially to mitigating ecophysiological disorders in the photosynthetic apparatus as the result of light stress, providing greater photochemical stability, survival and quality in the Lippia alba seed lings. No acclimatization process was required.