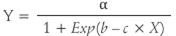INTRODUCTION
The cholupa or stone granadilla (Passiflora maliformis L.) is one of the eight cultivated species in the genus Passiflora, such as yellow passion fruits (Ocampo et al., 2015a), sweet granadillas (Ocampo et al., 2015b), and others with agronomic potential (Hurtado-Salazar et al., 2020). This species is native to northern Ecuador, Colombia, Venezuela and the Antilles and was introduced to Europe as an ornamental plant in greenhouses. The flower is hermaphrodite with a high degree of self-incompatibility (95%), and is cross-pollinated (allogamy) mainly by insects of the genus Xylocopa spp. (Ocampo et al., 2015a). The fruits are characterized by a high content of total phenols (277.00 mg gallic acid equivalent/L fresh weight-FW) and the total antioxidant activity (1,685.00 μmol Trolox/L FW) (Fischer et al., 2018). The cultivation of the cholupa has become a line of economic and social importance in the Department of Huila, Colombia, because of its high profitability and the generation of rural jobs, which can reach 648 wages per hectare for a three-year cycle. Cholupa is grown commercially in 14 municipalities with about 200 ha, Rivera being the principal one. In Huila, around 1,200 t of fruit were produced in 2018 (Agronet, 2019), of which 10% were pre or post-harvest losses, and 90% were destined for consumption in the regional market as fresh fruit, mainly in the preparation of juices. The production behavior throughout the year is continuous, with two marked harvest times: the first is October to January, the last two months having the highest production, and the second is from the first week of April to the end of May.
The effect of physical, chemical and biotic environments on the physiological mechanisms of a plant is known as plant ecophysiology (Larcher, 2003). The cholupa is cultivated in Colombia between 600-1,000 m a.s.l. (Ocampo et al., 2015a), with temperatures between 26 and 32°C, rainfall between 1,200 and 1,450 mm, relative humidity between 60 and 70%, and 8-11 h light/d (Fischer et al., 2018). These ecological conditions greatly affect the duration of the phenological stages of the plant (Fischer et al., 2009).
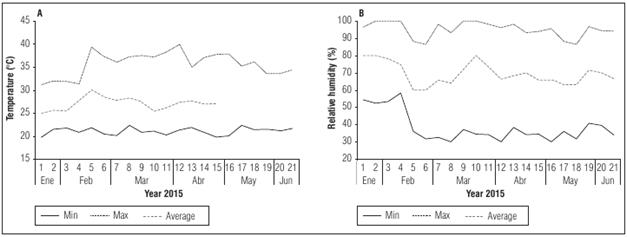
The study of biological events and their causes as a function of biotic and abiotic factors, as well as their relationship between the phases characterized by these events, within one or more species, is known as phenology (Silva et al., 2007). Phenological characterizations through the stages of the phenophases (vegetative and reproductive) provide greater detail in the description of plant cycle, helping in the prediction of seed collection for seed production and in the conservation and breeding programs of the species (Rego et al., 2006). Furthermore, the phenological identification of growth stages of mono- and dicotyledonous plant species uses the extended BBCH scale, an acronym of Biologische Bundesanstalt, Bundessortenamt und Chemical Industry (Meier, 2001). The scale has 10 main stages or plant development stages that are clearly identifiable and visible, starting with budbreak (stage 0) and ending with the latency period (stage 9); intermediate values include leaf development, buds, flowers, fruit development and subsequent ripening; secondary stages are also listed from 0 to 9 and are related to ordinal or percentage values of development (Mayor, 2011). Another useful tool to identify the physiological behavior of fruit species uses growth curves and development events, which are genetically determined, hormonally regulated and can be modified by environmental conditions (Garriz et al., 2005). They strengthen knowledge on a system (López et al., 2005), evaluate possible management strategies, and provide an approach for the potential yield (Cañizares et al., 2003). Among the non-linear models used to characterize growth and/or development as a function of time, the logistic, the exponential, and the monomolecular models stand out (García, 2008; Moreno-Medina et al., 2016; Almanza-Merchán et al., 2017).
Although there is an empirical knowledge from cholupa producers on the duration and the time (in the year) where the most interesting phenological events take place (Ocampo et al., 2015a), these events have not been described according to the BBCH scale, and there is no information on growth and development under this climatic condition. Therefore, the present study was proposed to identify the main phenological stages and approximate times of duration in order to generate a guide that estimates some growth and development parameters that help achieve integrated crop management.
MATERIALS AND METHODS
The study was carried out in the municipality of Rivera (02°44'29.4'' N, 075°77'19.5'' W), province of Huila (Colombia), located at 595 m a.s.l., during the period of December, 2014 to December, 2015. The fruit growth curve was used on a commercial crop in the municipality of Campoalegre (2°43'26.9'' N, 75°15'50.7'' W), located at 788 m a.s.l., with an average temperature of 27ºC and a relative humidity of 65%. The plants, 15 d old in a 4×4 m arrangement, were evaluated with a trellis system that consisted of a 2 m high mesh, on which the different types of crop branches were conducted and fixed. The climate of the region is classified as As according to Köppen, dry tropical area with a dry summer. For the characterization of meteorological conditions during the experiment, the climatological data, relative humidity (RH, %) and temperature (°C) were obtained from datalogger weather sensors (Onset HOBO UX100-003 humidity and temperature data logger, MicroDAQ, Contoocook, NH) every 6 h, with an accuracy of 3.5%. The precipitation variable was taken from climate databases (Wordclim).
Starting at 18 days after planting (DAP), the following variables were recorded weekly for 150 DAP in the selected plants: Length (cm) and number of nodes of the main stem and primary and secondary branches. Subsequently, for the fruit growth curve, ten flowers were marked in anthesis and weekly records were taken of the longitudinal and the transverse diameters of ten fruits per plant until ripening. the One plant was the sampling unit for the growth variables. The variables of each sample were used to model growth with the logistic function (1)
Where, α, the upper asymptote, is the maximum magnitude of the variable, c is the parameter that determines the slope of the curve, b is the moment when the maximum growth rate is achieved and X is the time (Seber and Wild, 1989). Based on these coefficients, obtained with STATISTIX 9, 2008, the growth curves for the plants established in the field were obtained. The BBCH-scale was used to identify the phenological development stages of the plants.
RESULTS AND DISCUSSION
Behavior of climatic variables
For the average temperature (Fig. 1A), an irregular behavior was observed with values between 21 and 30°C and marked peaks for the months of February and March, 2015 (39.4 and 37.3°C, respectively). An oscillating trend was observed for the relative humidity throughout the evaluation; the lowest values were in the month of February, 2015 (Fig. 1B). During this period, a high rate of vegetative growth of the stem and primary branches was detected.
Differences were observed between the daytime and nighttime temperatures, with fluctuations of 19.7°C; likewise, at night, the RH reached 99.8%, which was a positive condition for the occurrence of diseases, and, during the day, it dropped to 32.2%, with a difference of 67.7% between the 2 d. It should be noted that this low RH caused dehydration of the pollen and stigmatic fluid, reducing the fertilization process and facilitating flower abortion (Ocampo, 2013; Fischer et al., 2009).
Vegetative phase
During the vegetative phase, the development stages 1 (leaf development) and 3 (main stem elongation) were identified according to the BBCH scale (Meier, 2001). The times are presented in days after planting (DAP), averaging the date on which 50% of the plants reached each stage of development.
In the principal growth stage 1 (Tab. 1), it was possible to identify the secondary stages corresponding to the development of the third leaf (code 13) on day 6 DAP, along with the appearance of the fifth leaf (code 15) at 10 DAP, until obtaining nine true leaves (BBCH code 19) on 17 DAP. Afterwards, the development of new leaves continued, and it is possible to observe the appearance of the first tendril accompanying leaves, which had the characteristic climber habit of these species, one of the parameters that producers take into account when transplanting to the definitive place, along with the height of the plant.
Table 1 BBCH scale established for the growth and development of cholupa (P. maliformis) in Colombia.
The cholupa is characterized as a liana whose growth is continuous, with lateral flowering and basitonic branching (Tovar, 2009). During its development, lateral shoots begin to appear in the basal portion, below and above the knot with the first tendril.
Growth and development of the principal stem
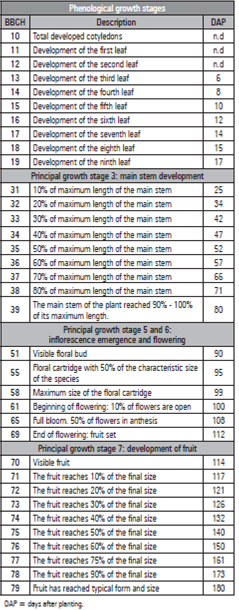
This plant is a climbing semi-perennial vine, with a cylindrical stem, glabrous or finely pubescent, green in color, striated, herbaceous and woody towards the base, with up to 12 cm in diameter (Ocampo et al., 2015a). At 23 DAP, the main stem reached an average of 37.03 cm. Starting at 30 DAP, the plants increased the growth rate, which, at 88 DAP, reached a maximum length of 455 cm, with a total of 68 nodes, presenting an emission rate of 2.8 nodes per week (Fig. 2) and an R 2 of 0.91 (Tab. 2). Schwartz (2013) stated that the phenological stages are good indicators of plant development rates, and, according to Angulo (2003), this behavior can be calculated with the period that lapses from node appearance to the appearance of the next node. At that time, pruning was carried out with cutting. The cut was made at the top of the node in order to activate lateral buds to stimulate the emission of the primary branches.

Figure 2 Length (A) and average number of nodes (B) of the main stem of cholupa (P. maliformis) plants.
Growth and development of primary branches
The branches can reach up to 30 m in length, with knots and internodes that form a lower bud, two linear, stipulated provisions (orange), a leaf and a tendril that provide plant support for the plant (Ocampo et al., 2015a). According to the typical behavior of passion flower crops, the growth of lateral shoots is successive, meaning that vegetative primary branches are generated from the main stem; afterwards, secondary branches originate from nodes of the primary branches, which, in the first production cycle, make up the group called “loaders or producers”, along with some tertiary branches, which must be thicker to support the weight of the reproductive structures that become fruit The curves for the length of the primary branching were sigmoid curves, characterized by a very rapid growth phase because of the budding response of the vegetative buds after emergence (Fig. 3).
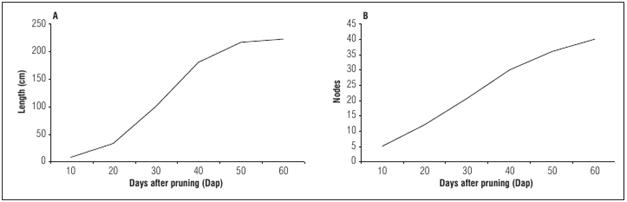
Figure 3 Length (A) and average number of nodes (B) of the primary branches of cholupa (P. maliformis) plants.
The maximum length of the primary branches (214 cm) was achieved near day 57 after pruning, along with the greatest number of nodes (35) in the evaluated period, with an emission rate of 0.86 nodes per day. After the emergence of the primary branches, the re-sprouting of secondary and tertiary branches begins because, physiologically, there is always a branch bud and the anterior buds break their dormancy.
Growth and development of secondary branches
The length and number of nodes of the secondary branches adjusted to the logistic model of three parameters over time; the highest average value was 138.4 cm, reaching maximum length approximately on 30 DAP (Fig. 4). The maximum number of nodes was achieved at 25-30 DAP (Fig. 4B), and the final number of nodes was 30. Thus, the rate of emission of nodes in the secondary branches in the initial phase (6-14 DAP) was 12 nodes per day, decreasing progressively, until reaching 0.9. This condition could be due to the presence of thrips (Neohydatothrips sp.) that caused damage to the terminal shoots and retarded branch growth but not the emission of nodes, resulting in branches with shorter internodes than healthy branches (longer internodes) (Santos et al., 2012).

Figure 4 Length (A) and average number of nodes (B) of the secondary branches of cholupa (Passiflora maliformis L.) plants.
The description of stage 39 with the elongation of the main stem (Tab. 1) included the maximum length of the main stem, 4.0 m, with a tutored system with an average height of 2 m. The grower allowed growth of the stem 2 m beyond the wiring before emergence and stimulated primary branch growth. Ten percent of the main stem growth of the cholupa was reached at 25 DAP on average, 50% was reached at 52 DAP, and 100% was obtained at approximately 80 DAP. The leaves are inserted on the stem using a petiole that is 2.3 to 8.5 cm in length with two subsesile glands (eventually two pairs), located in the lower half. The peduncle can have 3 to 9.5 cm in length and three bracts (cap) at its apex that are green and resemble leaves that are 5 to 8 cm long and 2.5 to 5 cm wide, which protect the flower and fruit in the stages of development (Ocampo et al., 2015a) (Fig. 5).

Figure 5 Principal growth stage 3: main stem development in cholupa (P. maliformis) plants. 5a) Stage 19, development of the ninth leaf. 5b) Stage 34, 40% of maximum length of the main stem. 5c) Stage 33, 70% of maximum length of the main stem. 5d) Stage 39, the main stem of the plant has reached 90% - 100% of its maximum length.
Reproductive phase
In the Passiflora species family, as in the cholupa, a stage of completely vegetative growth (juvenile phase of crop development) occurs with subsequent annual cycles of growth, where vegetative and reproductive stages occur simultaneously or slightly overlapping (Melgarejo et al., 2015). This fact results from the indeterminate growth habit of the crop, the origin and the adaptation to environments where it currently grows. The apex of growth of all branches is apt to form a floral primordium at the level of each node. The reproductive phase begins with the appearance of flower buds and ends with fruit ripening, in which the fruit has developed characteristics of appearance and texture, which is related to physicochemical changes in the pulp, such as flavor and aroma. Stages 5 and 6 include the appearance and development of the floral organ and flowering (Fig. 6 and Tab. 1). This process was evaluated on the secondary branches of the crop because it was the first cycle of production. The stages were identified from the emergence of the floral bud (stage 51), which is known as a floral cartridge until it reaches its maximum size (stage 58), where all parts of the flower are fully developed and the process of floral opening begins. The flower is generally alone, or rarely in pairs, pendular, pentamera, hermaphrodite, showy and with a pleasant aroma, with a length of 4.5 to 6.5 cm and a width of 4 to 5 cm, along with five petals and five sepals, lanceolate, reflexed, white and an interior mottled red-purple color (Ocampo et al., 2015a) (Fig. 6).

Figure 6 Principal growth stages 5 and 6: inflorescence emergence and flowering in cholupa (P. maliformis) plants. 5a) Stage 5. Stage 51: Visible floral bud. 5b) Stage 58, maximum size of the floral cartridge. d) Stage 6. Stage 61, Beginning of flowering: 10% of flowers are open and stage 65, full bloom. 50% of flowers in anthesis.
The full flowering (stage 65) and end of flowering or fruit set (stage 69) were also established. It should be noted that, in cholupa crops, the process of floral opening, pollination and flower closure takes place in 12 h, and the fruit set is estimated at approximately 2 days after anthesis (DAA).
The beginning of flowering is not uniform because plants originate from seeds, and the species is allogamous (Tovar, 2009). Flowering begins with the appearance of flowers on the lateral and basal branches. The flower, one per leaf axilla, is ephemeral, and anthesis takes only 12 h (Tovar, 2009). The first flowers in anthesis were observed at 108-110 DAP, and, at 180 DAP, the first ripe fruits were received. However, a high abortion of flowers was observed before anthesis, which was manifested by the presence of vain structures between the apex and the flowers. After this event, a new fall of flowers can be explained by natural abortion, excess humidity and/or lack of pollination, which turns into a decrease in production since it has been indicated that 67% of production and fruit quality depend on pollinators that decrease activity at that time (Tovar, 2009).
The BBCH codes with the respective description for stage 7: fruit formation (Fig. 7 and Tab. 1) was determined at 117 DAP, which is equivalent to a fruit between 7-10% of the final size, following the BBCH stages for fruit growth (stages 71-78), where 50% of the fruit size is reached at 140 DAP, 75% of the size is achieved by 161 DAP, 90% is seen by 180 DAP, and, finally, the maximum size on average is reached at 185 DAP. The fruits, upon maturation, did not have a color change, so abscission above the bracts that support it is used as an indicator of maturity.

Figure 7 Principal growth stage 7: Fruit development in cholupa (P. maliformis) plants. 7a) Stage 70, visible fruit. 7b) Stage 71, The fruit reaches 10% of the final size. 7c) Stage 74, the fruit reaches 30% of the final size. 7d) Stage 74, the fruit reaches 40% of the final size. 7e) Stage 77, the fruit reaches 75% of the final size. 7f) Stage 79, fruit has reached typical form and size.
Growth and development of fruits
The fruit is a berry in shape, spherical or ovoid, with an extremely hard shell (pericarp) (eventually soft) that is smooth and waxy, about 3.0 to 4.5 mm thick, with a white mesocarp (Ocampo et al., 2015a). Among the factors that determine development and final fruit size, Cavichioli et al. (2006) referenced genetic characteristics, temperature, number of flowers per plant and fruits in development; grower techniques could directly influence final size, such as irrigation, fertilization and pruning.
The analyzed variables showed a simple sigmoidal growth pattern (Fig. 8) as the result of the analysis with the fruit weight or size as a function of time (Hunt, 2003). The simple sigmoid pattern is consistent with that found in passion fruits (Arjona et al., 1991) and in gulupas (Lederman and Gazit, 1993). For each variable, the model with the best fit was chosen according to a more homogeneous distribution of the residuals, the highest coefficient of determination for prediction (R 2, Tab. 2), and the lowest mean square error.

Figure 8 Growth curves adjusted to a logistic model with three parameters of A, longitudinal and B, transverse diameters of the cholupa fruits.
The evolution of the longitudinal diameter was adjusted to the logistic model with three parameters, with an R 2 of 0.87 and a simple sigmoid growth pattern (Fig. 5A). Accelerated growth was observed until 15 DAA, where it tended to stabilize; a similar behavior was found in gulupas by Flórez et al. (2012), in passion fruits by Gómez et al. (1999), and in sweet granadillas by García (2008). The longitudinal diameter presented values very close to the transverse diameter (54 and 53 mm, respectively), which differed from that observed by García (2008) in sweet granadillas and reflected the characteristic spherical shape of cholupa fruits.
The increase in the transverse diameter of fruits was described with the logistic model with three parameters, with an R 2 of 0.91 (Fig. 5B) and presented a behavior similar to that of yellow passion fruits by Villanueva et al. (1999). In related species, the exponential phase occurs between 7 and 15 DAA, depending on the environment where the fruits grew; the growth rate decreased afterwards and tended to be almost constant after 20 d.
Based on the model adjusted to the fruit growth, it is possible to predict that harvest can be programmed 50 or 60 d after flowering since the longitudinal and transverse diameters tended to stabilize. The values estimated by the model explained each variable in relation to the field observations and, therefore, properly interpreted the physiological processes taking place in the fruits in each stage.
CONCLUSIONS
The vegetative and reproductive development phases of cholupa plants established in the province of Huila were described for the first time using the phenological BBCH scale.
The growth pattern of cholupas is a simple sigmoid type with three phases, which may vary slightly depending on the environmental conditions, relative humidity and precipitation.
The logistic model showed a good fit for describing the growth of the fruits under the specific conditions in the municipality of Campoalegre. These models can be used to schedule crop labor and predict the harvest time.