INTRODUCTION
Seeds play a fundamental role in nature as a means of dispersal and propagation of plants, but they are also important for their nutrient contents and as an important source of food for humans and other living beings (Bewley et al., 2013). From the agronomic point of view, seeds have high economic value because sexual reproduction is one of the most efficient and widely used methods in crop cultivation. Additionally, their economic importance depends on their being produced and marketed for food, industrial purposes, and medicine (Hartmann et al., 2014).
However, the functions of seeds at biochemical and physiological levels that determine fruit development and quality have been underlined to a lesser extent. Fruit set (an early stage of fruit development) is a consequence of fertilization of the ovum or ovules within the ovary; each fertilized ovum produces a seed, varying from one seed up to several hundred seeds per fruit in different species (Peña et al., 2010; Fischer, 2012; Yang et al., 2020). Fertilization first leads to the formation of seeds (with an exception of many parthenocarpic species); but it is, in turn, hormonal activity that triggers the ovary and, in some cases, other flower parts, to transform into a fruit (Agustí, 2004; Seymour et al., 2013). Double fertilization causes a local increase in auxin concentration resulting in the activation of gibberellin biosynthesis. The synchronized action of the two hormones controls the differentiation of the ovary into a fruit (Alabadí et al., 2009; Taiz et al., 2017).
Hormones synthesized in seeds (auxins, gibberellins, brassinosteroids, cytokinins, polyamines, ethylene, and others) regulate seed development (Sun et al., 2010; Kang et al., 2013) and increase the activity and strength of fruits as sink organs, a factor that determines fruit size (Fischer et al., 2012a). In this regard, in apples, increasing numbers of seeds result in an increased size of fruits (Sapir et al., 2017). Additionally, hormones influence fruit shape (Matsumoto et al., 2012; Sheffield, 2014) and, in some aspects, the final fruit quality (Hershkovitz et al., 2009, 2010, 2011). Seeds can also determine the abscission of fruits because, in case of normal seed development, fruits are more likely to remain attached to the plant until harvest (Agustí, 2013).
Knowledge and understanding of the functions that seeds perform for fruits are necessary to optimize agricultural tasks like fertilization, irrigation, pollination, pruning, application of phytoregulators, etc., that lead to improving the production and quality of fruit species. So, the objective of this review was to discuss the state of the art of the main functions of seeds during initiation fruit set, growth, development, and abscission of fleshy fruits, and to report the physiological and biochemical aspects that determine the seed-fruit relationships.
SEEDS
The seed is an organ of dispersion and propagation of angiosperms and represents the final trait of the reproductive evolution of plants (Azcón-Bieto and Talón, 2013; Taiz et al., 2017), although frequently the ovary wall or even additional flower parts could remain in close association with seeds, forming a more complex dispersal in grasses. In this way, seeds play a very important role in the life cycle of higher plants. Likewise, seeds are the basic food for humans and domestic animals and their value depends on the storage reserves of proteins, starch and lipids synthesized during seed development (Bewley et al., 2013). At some point of formation, the angiosperm seed is composed of embryo, endosperm, and seed coats (Agrawal and Rakwal, 2012). In order to initiate seed development, double fertilization must take place in which one sperm cell fuses with the egg cell to form a zygote, and the other one unites with the polar nuclei to give rise to the endosperm (Bewley et al., 2013).
After fertilization, the fruits and seeds undergo a concomitant development; however, in contrast to the fruit that can develop in the absence of pollination (Azcón-Bieto and Talón, 2013; An et al., 2020), seed development is more strictly dependent on double fertilization (Taiz et al., 2017; Ge et al., 2019). Seed development includes endosperm development and embryo growth, and both processes are regulated by various hormones such as auxins, cytokinins, gibberellins (GAs) and brassinosteroids (Sun et al., 2010; Hartmann et al., 2014), etc. In Citrus maxima (Burm.), during the early stages of seed growth, auxins, gibberellins and cytokinins are detected in complete seeds in higher quantities than in the small empty seeds (Yang et al., 2020), indicating the role of seeds as endogenous hormone centers maintaining fruit development.
FRUITS
Fruits are structures derived from an ovary (true fruit) or they can also include a variety of other tissues (false fruit). For example, the receptacle contains seeds and determines their dispersion (Seymour et al., 2013; Taiz et al., 2017). Fruits can be either dry or fleshy; fleshy fruits are the subject of interest in this review. They possess a juicy pericarp with abundant pulp and water and occasionally fibrous material. Fleshy fruits include drupes, berries, pomes, pepos and hesperidia (Cerri and Reale, 2020). After double fertilization the ovary undergoes cell division followed by cell expansion, a phase responsible for the final size of the fruit. In the expansion phase fruits undergo metabolic changes responsible for biosynthesis of many classes of metabolites including plant hormones (Paliyath et al., 2019). The final phase is fruit ripening, generally initiated once the fruit has acquired physiological maturity, at which stage it has attained the maximum size and capability for ripening, even after detachment from the mother plant (Paliyath et al., 2019). Many fruits show a single sigmoid growth phase such as lulo fruit (Almanza-Merchán et al., 2016), while others exhibit double or multiphasic growth patterns (Paliyath et al., 2019), like grapevine fruit (Almanza-Merchán et al., 2012; Almanza et al., 2012).
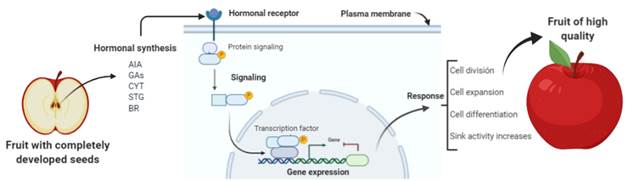
The formation and development of fruits have a close relationship to seed characters, such as the presence, number, size and distribution of seeds within the fruit. These are responsible for fruit set establishment, fruit retention on the plant, and the growth and quality of fruits (Fig. 1). These aspects, although considered essential, are poorly elucidated in fleshy fruit species, so that the objective of the present review is to address some of these issues in more detail.
Hormones, such as auxins (AIA), gibberellins (GAs), cytokinins (CYT), strigolactones (STG) and brassinosteroids (BR), are synthesized in the seeds developed within the fruit. They link to protein receptors in membranes, and trigger the signaling process that leads to changes in gene expression. As a result, the proteins being synthesized (structural and enzymatic) that are involved in cell division, expansion, differentiation and increase in source capacity, physiological processes affect the final quality of the fruits.
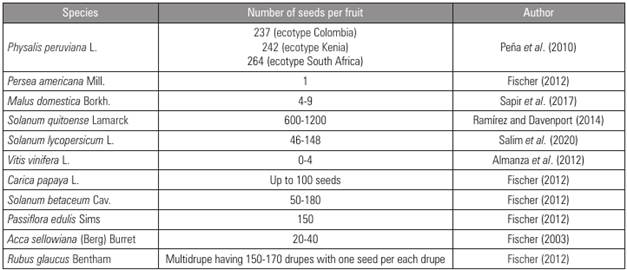
Although the number of seeds per fruit is variable and depends mainly on the species (Tab. 1), in some cases (such as for drupes and some berries) seed number may be sufficient to guarantee fruit growth of high quality, whereas, in other species, it is necessary that the fruit contain a high number of seeds, as in Solanaceae trees or in passion fruit (Fischer, 2012). However, there are certain exceptions to this trend, and one of these is the formation of parthenocarpic fruits, described in the following section.
Parthenocarpic fruits
Parthenocarpy is an alternative way for fruit set and growth. It consists of ovary development without seed formation (Agustí, 2013; An et al., 2020). Some examples of these species include cucumbers, bananas, grapes, citrus, pears, and apples (Agustí, 2013). Parthenocarpy can occur without fertilization or with pollination but without fertilization, or when both processes occur but the seeds abort (Gillaspy et al., 1993). It has been proposed that auxins serve as an alternative signal to replace pollination and fertilization to initiate fruit growth. Also, auxins interact with gibberellins (GAs) and both hormones stimulate cell division and expansion during fruit formation (Varoquaux et al., 2000; An et al., 2020). Furthermore, with a reduced activity of DELLA proteins the growth of parthenocarpic fruits can be promoted (Marti et al., 2007; Dorcey et al., 2009).
Obtaining seedless fruits is a desirable condition for consumers, like citrus without seeds, highly valued fruits for their ease of consumption (Iglesias et al., 2007). Likewise, fruit development without fertilization is advantageous in agronomy when the rate of fruit set is low. Pollen maturation and fertilization are affected by environmental factors such as temperature, light, and relative humidity. Unfavorable environmental conditions can dramatically decrease fertilization and, consequently, fruit development (An et al., 2020). Parthenocarpic plants can avoid this problem, allowing the development of seedless fruits under adverse environmental conditions for pollination and/or fertilization. In horticulture, parthenocarpy can be implemented to accelerate fruit production (Acciarri et al., 2002; Pandolfini, 2009). For research purposes, parthenocarpic fruits can be model fruits to understand the complex function of seeds in fruits. The application of 0.05% naphthalene acetic acid to the stigma of chili peppers under temperatures above 18°C during anthesis generated formation of seedless fruits, although these were smaller (Heuvelink and Körner, 2001). In tomatoes, eggplant, strawberry and raspberry, expression of auxin biosynthesis genes in ovary and ovules caused parthenocarpy (Mezzetti et al., 2004; Kataoka et al., 2009). The application of GAs can also generate parthenocarpy, as frequently occurs in apple and pear varieties that present difficulties in pollination (Agustí, 2013). This is because the exogenous GAs (for example, GA3) can generate changes in gene expression similar to those that occur with pollination (Vriezen et al., 2008).
In citric crops many varieties used for fresh consumption do not contain seeds, but fruit development is typical for seed-bearing varieties (Agustí et al., 2003). In these varieties, the stimulus of fruit growth cannot be attributed to seeds, but this process continues to be regulated by hormonal stimuli from the ovary walls (Agustí, 2013). In agreement with this, Mesejo et al. (2016) find that GAs are responsible for cell division during the set of parthenocarpic citrus fruits, and they conclude that, in species with autonomous parthenocarpy, GAs are responsible for reactivation of cell division in ovary during anthesis. The anthesis itself improves GA biosynthesis for maintaining cell division and probably acts as a feedback mechanism responsible for fruit formation.
Seedless fruits can possess better quality than fruits with seeds, for example when the latter have hard seeds or seeds of unpleasant taste, such as in Campomanesia lineatifolia, a berry whose seeds represent about 30% of the fruit weight (Balaguera-López et al., 2012). The seeds are not edible and hinder industrialization of the fruits. In eggplant, the absence of seeds prevents browning and reduction of pulp texture (Maestrelli et al., 2003). Additionally, seeds can produce substances that accelerate fruit deterioration, such as in watermelon and eggplant. So, the absence of seeds can increase the shelf life of fruits, allowing better conservation (Pandolfini, 2009). However, in tomato the fruits with seeds possess a higher beta-carotene content compared to parthenocarpic fruits, an important aspect from the nutritional point of view (Rotino et al., 2005; Salim et al., 2020).
ROLE OF HORMONES IN THE SEED-FRUIT RELATIONSHIP
Seeds and fruit set
Fruit set is the initial phase of fruit development during the sexual reproduction of flowering plants in which the flower parts transform into a fruit (An et al., 2020). Once the ovules that convert into developing seeds are fertilized, the ovary develops and transforms into the fruit (Taiz et al., 2017). The fruits and seeds undergo simultaneous development; however, some fruits can develop in the absence of pollination, a situation that cannot occur in seeds, with the exception of some cases of apomictic seed formation (Hojsgaard and Hörandl, 2019). Seed development is a process that has multihormonal regulation by auxins, cytokinins, GAs, brassinosteroids, and other hormones (Sun et al., 2010; van der Knaap and Østergaard, 2018). Likewise, the transition from ovary to developing fruit corresponds to fruit initiation, a process that is based on cell division and requires a large amount of energy. It is through hormonal synthesis that the developing fruit requires this energy in the form of carbohydrates (Azcón-Bieto and Talón, 2013). Therefore, the formation and development of seeds and fruits are hormonally regulated (van der Knaap and Østergaard, 2018).
Auxins, cytokinins and GAs immediately begin to regulate fruit set and development after fertilization. There is strong evidence to indicate that the role of auxin is facilitated by synergistic activity with GAs (Serrani et al., 2008; de Jong et al., 2009a). After fertilization, a seed produces an auxin signal (Mezzetti et al., 2004; Dorcey et al., 2009) that is believed to regulate the increment by biosynthesis of another hormone, the GA. This leads to the activation of GA signaling to the ovule to stimulate fruit growth (Dorcey et al., 2009), apparently through the degradation of DELLA proteins that repress the action of GA and, consequently, of growth (Kumar et al., 2013; van der Knaap and Østergaard, 2018). However, it appears also that the existence of independent DELLA protein pathways mediated by seeds contributes to fruit growth (Seymour et al., 2013).
It is important to mention that GA application to unpollinated ovaries triggers the initiation of fruit growth without affecting the expression of auxin signaling genes (Vriezen et al., 2008), while the development of fruits induced by auxin is significantly reduced by the simultaneous application of GA biosynthesis inhibitors (Serrani et al., 2008). These data suggest that auxins may act before GAs and that the effect of auxins is mediated at least in part by GAs. According to this hypothesis, the genes for GA biosynthesis are overexpressed after pollination and by auxin treatment of emasculated flowers (Serrani et al., 2008). However, in tomato fruits, each hormone seems to play a specific role; for example, the application of auxin generates a large number of cells in the pericarp, while the GA treatment reduces the number of cells in this part of the fruit, but causes cell development of a larger size (Serrani et al., 2007). Interestingly, a simultaneous treatment with both hormones results in the formation of fruits similar to the seed-bearing fruits obtained by pollination (Serrani et al., 2007), suggesting that GAs and auxins are necessary for normal fruit development (Crane, 1964; Agustí, 2013).
ARFs and Aux/IAA proteins also act during early fruit development, so that in Arabidopsis and tomatoes the expression of aberrant forms of ARF8 results in the formation of parthenocarpic fruits (Goetz et al., 2007) as well as the silencing of IAA9 in tomato (Wang et al., 2009). It is unclear by what mechanism ARF8 regulation occurs, but based on the model for auxin signal transduction, ARF8 and the IAA9 Aux/IAA protein may form a transcriptional repressor complex that is destabilized by aberrant forms of ARF8 to allow transcription of auxin response genes (Goetz et al., 2007). In tomato, pollination causes ARF9 upregulation and ARF7 repression, while the silencing of the SlARF7 gene in transgenic plants generates parthenocarpic fruits, which suggests that SlARF7 is a negative regulator of fruit set, and the silencing of this gene also results in a strong upregulation of SlGH3 encoding for an IAA-amido synthetase that can be induced to compensate for the excess of auxins (de Jong et al., 2009b; 2011).
Accordingly, most of the hormones related to fruit set are found in seeds (Crane, 1964). In fruits of sweet oranges, pollination increases GA levels in the ovaries of seed varieties (Ben-Cheikh et al., 1997). But the fact that varieties with similar fruit formation capacity, with and without seeds, have similar contents of gibberellins (Talón et al., 1990) indicates that these hormones must be responsible for the initiation of citrus fruit formation. The presence of auxins in pollen, their production in the style and the ovary due to fertilization and pollen tube growth, and the stimulation resulting from the ovary growth together with production of parthenocarpic fruits by exogenous application of auxins, indicate the function of this hormone during fruit set (Agustí, 2013).
In pears and tangerines, when seed formation is prevented by emasculation, the content of GAs in fruits is lower than in normally developing fruits, so that Agustí (2004) stated that this hormone contributes to initial fruit development. GA content in seeds is high, so that when seeds are formed after fertilization, they produce GA, which stimulates the synthesis of indole acetic acid (IAA) (Taiz et al., 2017). Imanishi and Hiura (1975) indicate that fruit set and fruit development are also promoted by cytokinins produced, along with auxins, in pollen, style, and seeds during pollination, fertilization, and seed formation. However, the effects of seeds on fruit set are probably associated with other factors that affect fruit set and growth, such as the position of fruits on the bunch, position of bunches on the plant, and environmental conditions (Kinet and Peet, 1997), etc.
Role of seeds in fruit growth and development
Pollination becomes an important process for yield (Tamburini et al., 2019), fruit growth and development, since it is one of the most decisive factors that determines seed formation. This is reported in custard apple (Annona cherimola), where a higher pollen load (twice that of the normal pollination) generated significantly greater pollen adhesion to the stigma, a higher number of seeds per fruit and, consequently, the fruits become larger (González et al., 2006). Pollination requirements in apple are lower than for some stone fruits; a commercial apple crop requires between 2 and 5% of blossom set compared with 80% for cherry. However, growers seek higher pollination rates to maximize fruit quality and market value (Pardo and Borges, 2020). The market favors to few large and well-shaped fruits rather than many small fruits (Park et al., 2018). This shows that pollination is an important process because it favors the formation of seeds, which in turn determines the quality of the fruits. So, in apple fruits cv. Anna, under conditions of the high tropics, a positive correlation was established between the number of seeds, fresh and dry weight of seeds, and fruit equatorial diameter (Cepeda, 2018).
Pollen grains have very high levels of brassinosteroids (Kanwar et al., 2017), hormones that in certain cases duplicate auxin functions and act on the expression of genes related to growth (Sun et al., 2020). But the possible action of brassinosteroids of pollen on fruit growth is unclear. In apples, a higher number of pollinated stigmas positively correlate with a higher number of seeds per fruit and a lower number of deformed fruits (Matsumoto et al., 2012). Likewise, the asymmetric fruits observed in low-set trees might be due to inadequate pollination (Fischer et al., 2012b). Increased pollen load improves the production and size of Japanese pear fruits (Pyrus pyrifolia), where concentrations of endogenous GA3 and GA4 increases in pollen tubes soon after germination and positively correlates with the final fruit size (Zhang et al., 2010).
Seed formation may be required for the synthesis and signaling of strigolactone hormones in the fleshy receptacles (commercially known as fruits) of wild strawberry (Fragaria vesca) (Wu et al., 2019). In particular, the expression of D27, MAX1 and LBO genes for the synthesis of strigolactones increases in receptacles after pollination, than decreases during the development of receptacles, and a low or null expression of these genes is detected in maturing fruits, suggesting involvement of strigolactones in early fruit development (Wu et al., 2019).
Thanopoulos et al. (2013) reports, for seedless chili fruits, considerably lesser weight of fruit and pericarp compared to fruits with seeds; the growth parameters such as length, diameter, fruit volume, and volume of fruit cavity are also lower in seedless fruits. Other results in the same species indicate that the seedless chili fruits are smaller, irregular in shape and show the carpeloid structures within the pericarp that generally makes them unacceptable for the market (Tiwari et al., 2007). In grapes, Friend et al. (2009) finds that the growth of seedless fruit is a result of the expansion of single cells, while that of a normal berries with seeds had both cell division and cell expansion.
Fruit size and shape
After fruit set, fruits grow due to the increase in cell size promoted by hormones synthesized by the developing seeds (Agustí, 2004). This suggests that adequate seed formation produces a large amount of auxins, GAs and cytokinins that leads to normal fruit development with optimal cell growth that in turn, determines the final fruit size (Kang et al., 2013). In grapes, endogenous hormones, such as auxins and GAs synthesized by the seeds, are transported to the pericarp and regulate its growth (Van-Huizen et al., 1996). Endogenous GAs in growing seeds highly correlate with fruit growth, so the larger the number of seeds, the greater the sink strength of the fruits for nutrients in the tree (Buccheri and Di Vaio, 2004) In kiwi, the metabolic pathways related to the synthesis and conjugation of IAA in developing seeds can influence high levels of auxin in the internal fruit tissues as well as on the amount of auxins exported from the fruits (Sorce et al., 2017).
According to Agustí (2004), the hormones synthesized in seeds play an important role in fruit growth, since hormonal activity influences the expression of genes, enzymatic activity, and membrane functionality. Most of the hormones produced in fruits are synthesized in seeds and exert their action through seed development, but they are also essential for the normal development of fruits; for this reason, the size and shape of many fruits are determined by the number and distribution of seeds (Srivastava and Handa, 2005). According to Sheffield (2014), the diameter and firmness of apple fruits do not depend so much on successful pollination per se, but on the resulting distribution of seeds within the fruit and the hormones that the seeds produce, that has an effect on fruit symmetry. Hormones produced by developing seeds in each carpel influence the growth of adjacent empty carpels; only when two or more adjacent carpels are empty does this reduce the fruit growth in this area, generating an asymmetric fruit (Sheffield, 2014). Some authors point out that the presence of cytokinins in fruits is a consequence of their transport from the roots, rather than their synthesis in developing seeds. Despite this, cytokinins are isolated from kiwi and lemon seeds (Agustí, 2013).
Brassinosteroids from immature seeds apparently stimulate fruit growth (Montoya et al., 2005). According to these authors, in tomato much higher levels of brassinolide are detected in the area of immature seed and coincide with the high activity of cytochrome P450 monooxygenase (CYP or P450) in this region of the fruit. This enzyme catalyzes the C-6 oxidation of 6-desoxocastasterone to castasterone, the immediate precursor to brassinolide, which is the most bioactive brassinosteroid (Montoya et al., 2005).
Despite evidence that the control of fruit shape is mainly exercised by plant hormones from seeds that stimulate growth to varying degrees, this is not true for all fruits. In bananas, for example, fertile seeds suppress the pulp development and, in this anomalous case, the failure of the fertilization allows the ovary to grow (Atwell et al., 2003). In tomatoes, the shape of the ovary regulates the spatial distribution of the seeds, which in turn, influences pericarp growth so that the size and shape of fruits become a function of the initial ovary shape plus subsequent fertilization and seed development (Razdan and Mattoo, 2006). Kojima (2005) affirms that the form that the fruit acquires at early stages of development is the effect of growth hormones produced by the immature seeds, of which, abscisic acid is found in high amounts in the pericarp, axils, and locular tissue at the early stages of fruit growth. Later on, large amounts of IAA appear, whose function is to determine the growth rate and shape of the fruit. However, expansion of the pericarp is not caused directly by the IAA but by the sink strength of the fruit caused by the developing seeds (Varga and Bruinsma, 1986).
In other Solanaceae, like the tomato, Agustí (2013) confirms that the IAA did not directly influence fruit development, but its action would be restricted to the seeds and involved in the growth of the embryo, where it creates a high sink strength, from which the pericarp grows. As the number of seeds increases, seed production of auxins per fruit also increases, and a strong basipetal flow of auxins results in developing high fruit capacity to attract mineral elements and photosynthates, and, thus, such fruits can grow more than fruits with few seeds (Friedrich and Fischer, 2000; Fischer, 2003). The developing seed is a strong sink for carbohydrates that are supplied as sucrose via phloem (Lu et al., 2020), favoring the final fruit weight (Peña et al., 2010). This confirms that seed formation is the integral component of fruit development (Gillaspy et al., 1993).
In grape cv. Merlot, an increase in fruit weight is based on the number of seeds per fruit, even if the fruits came from different parts of the bunch (Prudent et al., 2014). In cape gooseberry, this determines the content of seeds in fruits of three ecotypes, Colombia, South Africa and Kenya (Fischer et al., 2007); the fruits with the highest seed weight per fruit has a larger size, being the South African ecotype that generates the largest fruits (Peña et al., 2010). In tomato, Varga and Bruinsma (1986) observe that fruits with fewer seeds take longer to develop than normal fruits. In this species, a higher number of seeds per fruit results in increased fruit size (Bashir et al., 2018; Prudent et al., 2014). In agreement Crane (1964) proposes that the seeds serve as a focal point for mobilization of hormones and photosynthesis from the fruits.
In Capsicum flexuosum, the largest fruits have the highest number of seeds, with this number being greater when manual pollination is used (Carrizo, 2011). In the ‘Hass’ avocado, a strong correlation is found between seed size and fruit size (Garner and Lovatt, 2016). Seedless avocados are always smaller than seed-bearing fruits (Lovatt, 1990). In the ‘Arad’ avocado, there is a relatively large number of seedless fruits, together with those with seeds, making this fruit an excellent experimental model to study the implication of seeds for avocado seed quality (Hershkovitz et al., 2011). However, studies exist reporting little or no relationship (Garmendia et al., 2019) between fruit size and number of seeds (Chao, 2005; Gravina et al., 2011). And, although the seeds can improve the fruit diameter through a direct hormonal effect, an opposite side effect must be considered for the total yield per tree (Garmendia et al., 2019).
Seeds are centers of metabolic activity, and once their development has begun, they appear to have a predominant effect on fruit growth in many species (Grange, 1993; Kang et al., 2013). Picken (1984) concludes that the precise relationship between the fruit weight and the number of seeds in tomatoes can vary due to several factors affecting fruit weight, for example, through competition between the fruits, without affecting the number of seeds. In Cucurbita pepo, Stephenson et al. (1988) show that the seeds of the first fruit can affect the growth of the second fruit, apparently, this is because the auxins produced by the seeds of the first fruit inhibit the export of auxins from the second fruit and, therefore, decrease their sink strength. This explains the differential size of the fruits of the same bunch.
The number and distribution of seeds within a developing apple affects not only the weight but also the shape of the fruit (Brault and de Oliveira, 1995; Keulemans et al., 1996). Apples with shape defects are classified in lower priced categories (Musacchi and Serra, 2018), and fruits with a smaller number of seeds can grow deformed. One way to measure this abnormality is to use the asymmetry index; high values indicate increased fruit deformity. The asymmetry index increases in a curvilinear manner with an increasing number of well-formed seeds and fertilized ovules in the 'McIntosh' apples (Brault and de Oliveira, 1995). Similar results are observed in the 'Granny Smith' apple (Drazeta et al., 2004). The unequal forms of the fruits (asymmetry) can be determined by the amount of hormones (e.g. IAA and GA) derived from the seeds, generating an uneven distribution of nutrients and water within the pulp of the fruits (Matsumoto et al., 2012).
Seeds and fruit maturation and quality
Seeds are not only involved in the determination of the size and shape of fruits, but can have an effect on fruit ripening and quality. In this regard, in the atemoya, the absence of seeds contributes to the smaller size of the parthenocarpic fruits and less calcium accumulation in the pericarp, making the fruits less firm (dos Santos et al., 2019). Hershkovitz et al. (2011) found that seedless 'Arad' avocado fruits have less firmness, confirming the importance of seeds in fruit development and quality. Hershkovitz et al. (2010) mentions that seeds in avocado fruits are involved in delay of the ripening processes. Seedless avocado fruits ripen more quickly both in refrigeration and at room temperature, due to a faster start of the climacteric production of ethylene, significantly higher respiratory rate, and a greater softening of fruits (Hershkovitz et al., 2009). In the 'Arad' avocado the seeds increase the sensitivity to ethylene of mesocarp that surrounds the seed during the late ripening process (Hershkovitz et al., 2009). Hershkovitz et al. (2011) states that seedless avocado fruits have a higher relative expression of genes that code for CTR1 throughout development and ETR during ripening. This suggests the important role that seeds play in inhibiting the ethylene-induced gene response. Previous studies in this species suggest that the seeds delay the onset of climacteric stage in mature fruits (Hershkovitz et al., 2009). Thanopoulos et al. (2013) reports that seedless pepper fruits have a lower respiratory rate but a higher reddish coloration.
Seed development is necessary for cell division within the grapefruit mesocarp (Friend et al., 2009). In apples, the best pollinated fruits contain more seeds, are larger, and generally have better external quality characteristics, but this only occurs if the number of fruits per tree are optimal (Blažek and Hlušičková, 2006). The high number of seeds seems to accelerate maturation but decreases susceptibility to fungal rot, apparently, due to the increase in the contents of phenolic compounds (Blažek and Hlušičková, 2006). High seed numbers were also associated with higher calcium content, increased fruit firmness and acidity (Tomala, 1999; Buccheri and Di Vaio, 2004). Parthenocarpic varieties of apple have greater susceptibility to calcium deficiency (Bangerth, 1976), indicating that the number of seeds is an important factor that determines the calcium content in fruits at harvest. Bramlage et al. (1990) finds that apple with a higher number of seeds have a larger diameter and a higher concentration of calcium, while with magnesium and potassium there is no such correlation. These authors also find that the internal browning of the fruits increases when the number of seeds is lower, an aspect related to a lower calcium content (Bramlage et al., 1990).
Similar results are found in grapes by Boselli et al. (1995), who report that fruits with 4 seeds are larger and have a higher concentration of Ca, K and Mg compared to fruits with a lower number of seeds. In this regard, Kubowicz et al. (1982) mention that a higher number of seeds per fruit can result in greater transport of auxins from the fruit, which, in turn, can improve the transport of Ca. Copeland and McDonald (2001) indicate that lesser solar radiation results in smaller seeds, due to a reduction in photosynthetic rate that reduces the period of seed filling. In apple trees, as the number of seeds per fruit increases, the fruit size and calcium concentration increases (Bramlage et al., 1990).
Seeds and fruit abscission
The effect that seeds have on premature fruit fall is well known in many species, where null or inadequate pollination and/or fertilization can generate early fruit fall (Agustí, 2013). In fruits with varieties that have seeds, the synthesis of GA in the fertilized ovule is the factor that controls initial fruit development (Talón et al., 1990), so that its elimination or emasculation, which prevents seed formation, stops fruit development and provokes its abscission. However, in these cases, an application of GA restores growth (Agustí, 2004). Furthermore, the use of a gibberellin synthesis inhibitor, paclobutrazol, causes fruit abscission (Ben-Cheikh et al., 1997). This evidence suggests that endogenous GAs are the main responsible factors for fruit set in citrus, but the regulation of ovarian development cannot be attributed exclusively to the seeds (Agustí, 2004). In 'Hass' avocados, a large proportion of fruits are abscised due to the lack of pollen germination and subsequent fertilization; the abortion of ovules and reduction of fruit growth preceding the fruit abscission are related to the accumulation of abscisic acid in fruit tissues (Garner and Lovatt, 2016). These same authors mention that the demonstration of the changes that occur in seed development and hormone concentrations of abscising and persisting fruit during key stages of ‘Hass’ avocado fruit development will provide fundamental information required for developing and implementing horticultural strategies for decreasing abscission and increasing yield (Garner and Lovatt, 2016).
Persistence and fruit growth are related to seed presence that is demonstrated by existence of development regulatory substances synthesized in seeds (Agustí, 2004). The responsiveness of avocado seedless fruits to ethylene could explain why these fruits have a greater tendency for abscission than seed-bearing fruits (Davenport and Manners, 1982; Ramírez and Davenport, 2014). An apple fruit that contains only a few seeds is more likely to fall from the tree before reaching maturity, especially if water, nutrients, or carbohydrates are scarce (Mantinger, 1997).
CONCLUSIONS
The fruit is a mature ovary that has the functions of formation, protection, and dispersion of seeds, with some exceptions as in the case of parthenocarpic fruits. The seeds, in turn, become an important determinant of fruit development. After pollination and fertilization, seed development begins, and, in turn, the ovary (and, in some cases, the accessory tissues) initiate fruit formation. The hormones produced by seeds are responsible for ensuring the initiation of the fruit, which continues to grow, acquires quality-determining characteristics, and does not fall prematurely. Fruits with an adequate number of developed seeds has better size, shape, and quality. This relationship between seeds and fruits is and must be an important factor to consider for agronomic management of fruit species worldwide in order to guarantee their success. But there are still biochemical and molecular physiological aspects that are unknown and must be studied in order to understand with greater certainty the complex seed-fruit relationship.