INTRODUCTION
Zingiberaceae Martinov is the largest family of the order Zingiberales, with 52 genera and about 1,600 species (WFO, 2019). The family has a pantropical distribution, with only one genus (Renealmia L.f.) distributed in the Neotropics and Africa, while four others (Alpinia Roxb., Curcuma L., Hedychium J. Koenig and Zingiber Mill.) were introduced into and naturalized in the Neotropics (Kress, 1990; Stevenson and Stevenson, 2004; Hamidou et al., 2012). In Brazil, the family is represented by eight genera, 32 species and one subspecies (BFG, 2015, JBRJ, 2020).
The floral morphology of the family invariably consists of bisexual and zygomorphic flowers with a normally tubular calyx, formed by two sepals, a corolla with three petals and two fused petaloid staminodes, forming an abaxial labellum, and a functional stamen with two thecae (Kirchoff, 1988; Endress, 1994). Additionally, more than 25 genera in this family have a crest at the anther apex, with broad variation in shape and size (Fan and Li, 2012; 2016).
The anther crest is a specialized anther appendage that extends up from the top of the anther to form a tail-like structure, with broad variation in shape and size between the different genera, and is widely characterized in the Zingiberaceae family. The function of the anther crest is to boost the male and female functions of plants, directing pollinators to an ideal position for removing and receiving pollen (Fan and Li, 2016).
The style is thin, and the anther fillet is inside a ventral groove between the thecae. The fusion of the lateral staminodes into a labellum, the presence of a pair of nectariferous glands at the style base, and cells containing essential or ethereal oils are autapomorphies in this family (Kress, 1990).
The peculiar floral morphology of the family Zingiberaceae may be related to a specialized floral system. Flexistyly is a sexual dimorphism consisting of two forms that differ in the temporal expression of sexual function and that involve the reciprocal movement of the stigmatic surface through a vertical axis during the flowering period, which combines reciprocal herkogamy and dichogamy and promotes outcrossing (Li et al., 2001; Cardoso et al., 2018). This strategy has been documented in 24 Zingiberaceae species, including those within the genus Alpinia (Kress et al., 2001).
Alpinia is the largest genus of the family Zingiberaceae, with 346 widely distributed species in tropical Asia (Smith, 1990; Kress et al., 2001). The following three species are frequently cultivated in Brazil: A. purpurata (Vieill.) K. Schum., A. vittata W. Bull, and A. zerumbet (Pers.) B.L. Burtt & R.M. Sm. (JBRJ, 2020). Studies based on molecular data have suggested that this genus is highly polyphyletic (Kress et al., 2001), and most authors consider it to be taxonomically difficult and complex (Smith, 1990; Kress et al., 2001).
Apart from an important ecological role in the understory of tropical and subtropical forests, Alpinia species can be used as medicinal plants (e.g., A. officinarum Hance), as food (A. galanda (L.) Willd.) or for landscaping purposes (A. zerumbet), mainly as cut flowers, as is A. purpurata (Althaus-Ottmann et al., 2011), which has varied color bracts and great ornamental value for decorative purposes in gardens and landscapes because of the durability and exuberance of its inflorescences with year-round flowering.
Reproductive biological studies are essential for the cultivation, conservation, and genetic development of plants (Baskorowati et al., 2010). Knowledge on the reproductive barriers of Alpinia helps the development of research on this promising ornamental plant. The main goal of the present study was to examine the floral morphology and morphometry of four A. purpurata cultivars and to analyze their reproductive system, focusing on pollen and stigma viability.
MATERIALS AND METHODS
Study location
The active germplasm bank (BAG) of the Universidade do Estado de Mato Grosso in the municipality of Tangara da Serra, MT-Brazil (14º39 'S, 57º25' W; 321 m a.s.l.) was the study site for the four A. purpurata cultivars (Jungle King, Kimi, Pink Ginger and Red Ginger) planted in August, 2015. The regional climate is tropical, with a dry season from May to September and a rainy season from October to April, and an average annual rainfall of 1,830 mm (Dallacort et al., 2011). The soil was classified as Latossolo Vermelho Distroférrico, clayey, with a flat to slightly undulating topography (Embrapa, 2018).
Floral morphology and morphometry
The morphological characteristics were evaluated and illustrated with drawings of flowers during the anthesis period, detailing the external morphological characteristics of A. purpurata flowers. Photomicrographs were taken with a Leica DMLB photomicroscope equipped with a Zeiss Axio Cam digital camera.
The floral morphometry of the four A. purpurata cultivars (Jungle King, Kimi, Red Ginger and Pink Ginger) was analyzed in fresh flowers in anthesis (N = 25). A pachymeter was used to measure the flower length and width.
Statistical analyses were performed using Sisvar® (Ferreira, 2000). The means and standard deviation of the floral structures of the four A. purpurata cultivars were compared by the Tukey test.
Floral biology
Floral anthesis, pollen viability and stigmatic receptivity were observed in 40 flowers from 10 plants per cultivar at flowering peak, from March to May of 2016. During anthesis, the flowers were monitored from the beginning of opening until senescence, characterized by a loss of glossiness and darkening of the flower.
Pollen viability was determined in previously bagged flowers in anthesis. The flowers were evaluated every two hours, from 07:00 AM to 5:00 PM. At each sampling, five slides per cultivar were mounted. The pollen grains were stained with acetic carmine, and 200 units per slide were counted with an optical microscope (Kearns and Inouye, 1993). Grains with stained protoplasm were deemed viable, and those with a translucent and ruptured envelope were deemed unviable.
The stigmatic receptivity of the flowers used in the pollen viability tests was evaluated with two solutions: alpha naphthyl acetate, in which the reaction with esterase darkens the stigma if it is receptive, and hydrogen peroxide (H2O2), which, through the presence of the enzyme peroxidase, indicates receptivity by forming air bubbles (Kearns and Inouye, 1993). In addition to the stigmatic region, the solutions were also used to test the receptivity of the anther crest located just above the stigma because the presence of stigmatic fluid in this structure facilitates the reception of pollen grains.
Breeding system
Pollination treatments
Preliminary observations confirmed the receptivity of the anther crest in the four A. purpurata cultivars. Therefore, controlled pollination was carried out with three methods: (1) simultaneously on the stigma and anther crest, (2) only on the stigma, and (3) only on the anther crest.
The flowers of all cultivars, previously protected with organza bags, were hand-pollinated between 07:00 and 09:00 AM. After pollination, the inflorescences were bagged again and monitored until flower senescence or fruit formation.
The previously bagged inflorescences and floral pedicels of the four A. purpurata cultivars were pollinated as follows: (1) spontaneous self-pollination, bagging inflorescences with intact flowers; (2) hand self-pollination of previously bagged inflorescences with the manual transfer of pollen to stigma on the same flower; (3) geitonogamous pollination of previously bagged inflorescences with pollen transfer between flowers on the same plant; (4) cross-pollination of inflorescences with pollen transfer between flowers from different cultivars; (5) natural pollination (control) of inflorescences exposed to pollinators and monitoring of fruit formation.
The criteria for the selection of cultivars for cross-pollination were chosen with the ornamental plant market in mind, such as variations in the color of the bract and productivity among cultivars.
The low number of flowers in the Red Ginger and Pink Ginger cultivars was possibly due to investment in the aerial plant parts (bulblets) in the bract axils.
Pollen tube growth
The pollen tube growth was analyzed in the ventral region of the anther crest. The flowers were collected 1 d (approximately 24 h) after manual and natural pollination and fixed in FAA for approximately 36 h. For slide preparation, the flowers (N=25 flowers per cultivar) were rinsed three times in distilled water and treated with sodium hydroxide (8N NaOH). The pistils were then mounted on slides and stained with 0.1% aniline blue solution at 0.1 mol/L K3PO3 (potassium phosphite). The pollen tube growth was observed with an Olympus BX 51 epifluorescence microscope.
RESULTS AND DISCUSSION
A. purpurata inflorescence is terminal, globose or spiciform, and the flowers are protected by colorful and conspicuous bracts (Fig. 1A, 2A). Five to seven flowers or vegetative propagules (bulblets) emerge from each bract. The flowers are tubular, bisexual, zygomorphic, and white (Fig. 1B-C, Fig. 2 A-B) and have a fused calyx, a tubular corolla with three petals fused with the curved distal lobes, one fertile stamen with a conspicuous anther grooved ventrally with the apex crest (Fig. 1D), and four external lateral staminodes, two on either side, forming a petaloid labellum (Fig. 1E). The stigma is exserted above the anther, flattened (claviform) and moist, with a ciliate margin (Fig. 1E). The upper part of the ovary has two epigynous nectaries surrounding the style base (Fig. 1F). The ovary is tricarpellary, trilocular and plurilovular. The fruit has a 3-valved dry or fleshy capsule, dehiscent from apex to base, containing numerous toothed seeds.
The morphometric calyx and corolla characteristics of the Red Ginger and Pink Ginger cultivars were similar, while the other cultivars differed significantly from each other in this regard (Tab. 1).
Table 1 Flower morphometry of four Alpinia purpurata cultivars.
| Cultivars | Jungle King | Kimi | Red Ginger | Pink Ginger |
|---|---|---|---|---|
| Floral structure length (cm) | X ±SD | 𝑿 ±SD | 𝑿 ±SD | 𝑿 ±SD |
| Calyx | 2.38 ± 0.11 a | 2.16 ± 0.14 b | 1.65 ± 0.06 c | 1.88 ± 0.08 c |
| Corolla | 4.68 ± 0.15 a | 4.30 ± 0.16 b | 3.10 ± 0.17 c | 3.48 ± 0.20 c |
| Lacinia of the corolla | 1.42 ± 0.10 a | 1.38 ± 0.09 a | 1.00 ± 0.06 b | 1.16 ± 0.04 b |
| Labellum | 1.32 ± 0.05 a | 1.22 ± 0.08 a | 0.96 ± 0.02 b | 1.04 ± 0.07 b |
| Stigma | 4.12 ± 0.12 a | 3.47± 0.16 b | 2.53 ± 0.20 c | 2.93 ± 0.20 b |
| Anther + crest | 0.97± 0.02 a | 0.88 ± 0.02 b | 0.68 ± 0.03 c | 0.75 ± 0.02 d |
| Ovary | 0.60 ± 0.04 a | 0.65 ± 0.06 a | 0.57 ± 0.09 a | 0.58 ± 0.02 a |
Means followed by the same letter in a row do not differ from each other according to the Tukey test at 5% probability; n = 25 flowers.
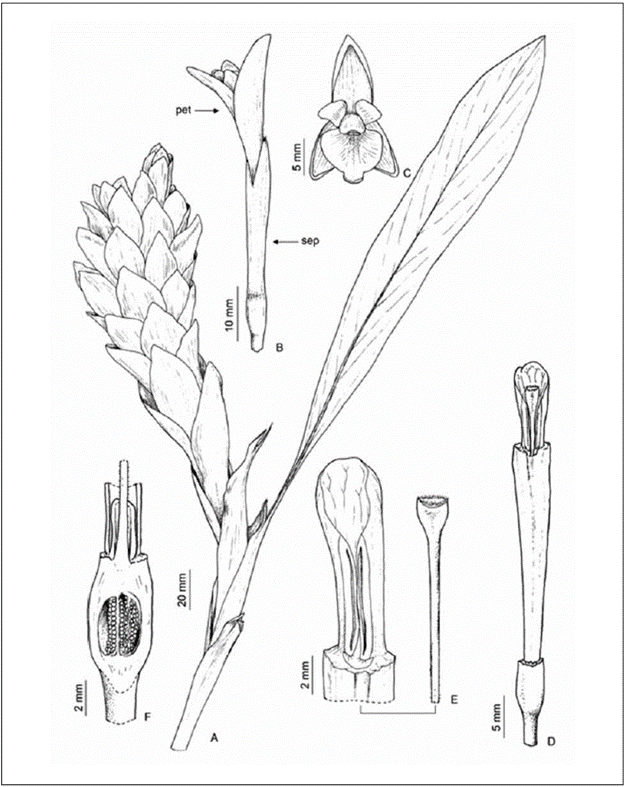
R. Pinto
Figure 1 Terminal inflorescence of A. purpurata; A) stem apex with inflorescence; B) side view of the flower; C) front view of the flower; D) detail of the tubular corolla; E) detail of stigma and anther; F) inferior ovary with nectariferous glands at the fillet base.
Table 2 shows the comparisons between the lengths of the floral structures. The length and labellum of the corolla lobes were proportional to the calyx length and corolla of the Jungle King and Kimi cultivars. The anther length and crest differed statistically between the cultivars and were related to corolla length. The ovary length did not differ significantly between the cultivars.
The different flower sizes observed in the four A. purpurata cultivars may have been the result of environmental differences, such as temperature and relative humidity, as recorded for other Alpinia species (Takano et al., 2013).
As observed for A. purpurata, there are many other plants in which all or some flowers of an inflorescence are converted into asexual bulbs: Polygonum viviparum L. (Polygonaceae) (Diggle, 1997), Globba spp. (Zingiberaceae) (Box and Rudall, 2006), and Calathea marantifolia Standl. (Marantaceae) (Matlaga and Horvitz, 2009). According to Box and Rudall (2006), the production of bulblets allows herbaceous plants to grow vigorously and ensures fast establishment.
The anthesis period, characterized by the opening of the corolla lacinia in the four A. purpurata cultivars lasted 9 to 12 h. Anthesis in the Jungle King, Red Ginger, and Pink Ginger cultivars started at 06:00 AM, and, in the Kimi cultivar, it started at about 09:00 AM. Anthesis ended between 05:00 and 06:00 PM on the same day. At the beginning of anthesis, the stigma was positioned above the anther, which was dehiscent. Unfertilized flowers were not abscised during the evaluation period. The ovary development of the fertilized flowers began about 4 d after pollination. The bracts remained attached to the inflorescence throughout fruit maturation. Style movements, such as ''anaflexistylous'' and ''cataflexistylous'' phenotypes, were not observed.
The stigmatic fluid was continuously secreted during flowering, forming a drop on the stigma, which could reach and cover the anther crest (Fig. 2C-D). In Roscoea debilis (Zingiberaceae), the stigmatic fluid aids germination of pollen grains and pollen tube elongation in the styles (Fan and Li, 2012).

Figure 2 External morphology of Alpinia purpurata. A) Inflorescence with presence of flowers in the bracts; B) front view of flower; C) anther crest (arrow) and dehiscent anthers; D) side view of the anther crest with exudate release (arrow) at the stigma.
The receptivity of the stigma and anther crest was confirmed by the response to the alpha-naphthyl dye and the H2O2 solution (Fig. 3A-B). A pollen viability of more than 86% was observed in all cultivars during the evaluation period. The viability peak was between 07:00 and 11:00 AM (Tab. 2). Although the A. purpurata stigma was receptive when viable pollen was released, the stigma position above the anther hampers selfing in this species. The floral herkogamy prevented pollination within a flower but not between plants of the same cultivar.
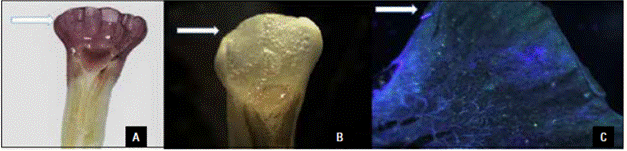
Figure 3 Receptor reaction in the anther crest and stigma of Alpinia purpurata (arrow). A) Receptivity using alpha naphthyl dye; B) receptivity using hydrogen peroxide; C) pollination in the anther crest underepifluorescence after 36 h.
Table 2 Pollen viability (%) of four Alpinia purpurata cultivars.
| Time of day/ | 7:00 𝑋 ±SD | 9:00 𝑋 ±SD | 11:00 𝑋 ±SD | 13:00 𝑋 ±SD | 15:00 𝑋 ±SD | 17:00 𝑋 ±SD |
|---|---|---|---|---|---|---|
| Cultivars | ||||||
| Jungle King | 94.5±2.6 | 94.1±3.8 | 94.5±2.1 | 93.8±2.8 | 89.7±3.5 | 87.9±6.8 |
| Kimi | - | 95.1±1.7 | 94.9±1.4 | 95±1.4 | 89.3±6.5 | 87.8±6.5 |
| Red Ginger | 94.9±3.6 | 95.3±1.3 | 94.9±2.9 | 90±4.1 | 88.3±4.7 | 86.6±6.1 |
| Pink Ginger | 93.6±2.1 | 93.4±3.2 | 93.8±2.0 | 93.1±2.8 | 89.9±4.0 | 88.1±4.8 |
n = 25 flowers.
The fruiting percentage in the cross-pollination treatments ranged from 60 to 83% between the cultivars (Tab. 3). The number of seeds/fruit with manual cross-pollination was 22% lower for the Kimi cultivar than for the other cultivars (Tab. 3).
No full pollen tube growth in the anther crest tissue was recorded in any of the hand-pollinated treatments (Fig. 3C), and no fruiting was recorded after a manual pollen transfer in the anther crest region, indicating that this structure must act only in the reception of pollen grains that germinate and emit pollen tubes that grow to the stigma through the stigmatic fluid. The simultaneous pollen transfer to the stigma and anther crest regions in the manual cross-pollination treatments caused a 45 and 52% increase in seeds/fruit in the cross for the Kimi and Jungle King cultivars, respectively, as compared to manual cross-pollination only performed on the stigma (Tab. 3).
The treatments with spontaneous self-pollination, hand self-pollination and geitonogamy did not induce fruiting, indicating obligate xenogamy. Therefore, A. purpurata is an allogamous and self-incompatible species (Tab. 3).
The mating system of A. purpurata cultivars is exclusively xenogamous. The obligate xenogamy facilitates the breeding of new hybrids for the ornamental plant market (Luc-Cayol and Fereol, 1997). Flexistyly has been observed to promote outcrossing by avoiding self-pollination (Li et al., 2001). The absence of flexistyly in A. purpurata may be a result of the effectiveness of self-incompatible and herkogamy at preventing autogamy, with no need for another mechanism to promote cross-pollination. Self-compatibility with pollinator dependence has been reported in flexistylic Alpinia species (Li et al., 2002; Zhang et al., 2003; Krieck et al., 2008).
The lower number of fruits with the natural pollination than with the artificial pollination suggested that there was a limited presence of effective A. purpurata pollinators in the study area.
In the A. purpurata, although hand pollination of the anther crest did not induce fruit formation, this structure above the stigma, together with the stigma, seemed to play an important role in the reproductive biology of this species. Because of its physical proximity to the stigma, the anther crest is covered by stigmatic fluid. The data indicated that this structure helps to capture pollen grains, functioning as a stigmatic extension that must touch the pollinator's body at the time of the visit. Pollen grains that adhered to the anther crest reached the pistil because there was an increase in seed production when pollen was deposited on the structure, evidencing participation in the pollination process. In addition, the crest protects against the viscous secretion (stigmatic fluid) produced and stored at the apex of the stigma. In Costus (Zingiberales-Costaceae), this structure has the same function in pollination (Araújo and Oliveira, 2007).
In Roscoea debilis (Zingiberaceae), the stigmatic fluid forms a large globule and seeps into nearby pollen grains, inducing pollen germination and pollen tube elongation into the style. This process ensures reproduction when pollinators are limited (Fan and Li, 2012), which was not observed in the present study.
Similar to other Zingiberaceae species (Sakai et al., 1999), A. purpurata maintains the characteristics of insect-pollinated flowers, namely white, zygomorphic flowers with a landing platform and nectar rewards.
Table 3 Reproductive system of four Alpinia purpurata cultivars.
| Pollination on stigma + anther crest | ||||
|---|---|---|---|---|
| Treatments | Cultivars | No. of flowers | Fruiting percentage (%) | No. of seed/fruit 𝑋 ±SD |
| Cross pollination | Kimi¹ x Jungle King² | 25 | 60.0 | 200.4±57.1 |
| Jungle King¹ x Kimi² | 25 | 76.0 | 203.4±62.2 | |
| Red Ginger¹ x Pink Ginger² | 8 | 87.5 | 166.0±54.6 | |
| Jungle King¹ x Pink Ginger² | 6 | 83.0 | 158.6±51.3 | |
| Pollination on anther crest | ||||
| Cross pollination | Kimi¹ x Jungle King² | 25 | 0.0 | 0.0 |
| Jungle King¹ x Kimi² | 25 | 0.0 | 0.0 | |
| Pollination on stigma | ||||
| Cross pollination | Jungle King¹ x Kimi² | 25 | 80.0 | 105.5±4.8 |
| Kimi¹ x Jungle King² | 25 | 24.0 | 93.5±20.9 | |
| Geitonogamy | Jungle King | 25 | 0.0 | 0.0 |
| Kimi | 25 | 0.0 | 0.0 | |
| Spontaneous selfing | Jungle King | 25 | 0.0 | 0.0 |
| Kimi | 25 | 0.0 | 0.0 | |
| Hand selfing | Jungle King | 25 | 0.0 | 0.0 |
| Kimi | 25 | 0.0 | 0.0 | |
| Natural pollination | Jungle King | 𝑋 =288* | 10.0 | 178.9±34.0 |
| Kimi | 𝑋 =280* | 0.0 | 0.0 | |
| Pink Ginger | - | - | 0.0 | |
| Red Ginger | - | - | 0.0 | |
1 In the crosspollinated treatments, pollen receptor; 2 X pollen donor.
* Mean number of flowers produced per inflorescence within two months, counted from the scars of the floral pedicel in the inflorescence.
CONCLUSION
During the anthesis period, the anther's stigma and crest had receptivity, indicating that both structures were covered by stigmatic fluid. The A. purpurata flowers did not have stylet movement (flexistyly).
The cultivars had fruiting with the cross-pollination. Low fructification occurred with the natural pollination, and there was no fructification with the spontaneous self-pollination, manual self-pollination or geitonogamy.
Outcrossing in A. purpurata facilitates new natural hybrids for the ornamental plant market.















