INTRODUCTION
Surinam cherry, Brazilian cherry or Cayenne cherry (Eugenia uniflora L.), belongs to the Myrtaceae family and is a native plant species of the tropical east coast of South America, with dispersion from Suriname to southern Brazil (Lorenzi and Matos, 2008). The essential oil produced in the leaves is suggested as an important raw material for the cosmetic, pharmaceutical and perfumery industries because of its antioxidant effect and antimicrobial and antihelmintic ativities (Amorim et al., 2009; Gallucci et al., 2010; Victoria et al., 2012; Rodrigues et al., 2013; Santos et al., 2018).
According to Melo et al. (2007), the major constituents in Surinam cherry essential oil are furanodiene and its rearrangement products, furanoelemene (or curzerene, 50.2%), β-elemene (5.9%) and α-cadinol (4.7%). However, the essential oil composition of this species is reported as greatly varied in the literature, associated with phenotypic characteristics (Costa et al., 2010) and edaphoclimatic conditions (Costa et al., 2009; Rodrigues et al., 2013; Santos et al., 2018; Silva et al., 2018).
Because of the high variability found in the essential oil of Surinam cherries during the different seasons of the year and also because it is a semi-deciduous species, the development of drying techniques allows Surinam cherry leaves to be stored and marketed throughout the year. The drying of aromatic plants can be natural, which is generally carried out under shade and at ambient temperature, or done with artificial driers, with or without ventilation (Rocha et al., 2011). The drying process preserves a plant’s active components by reducing the oxidation process and microbial growth (Harbourne et al., 2009; Voung et al., 2015).
The drying method can also affect the essential oil yield of aromatic plants, as demonstrated by Amaral et al. (2018, 2019) in native Brazilian species of the Myrtaceae and Asteraceae families. Choosing the drying method is particularly important for aromatic plants since it can affect essential oil yield and composition according to the plant species and the secretory structure where the oil is accumulated (Rahimmalek and Goli, 2013).
Some plant species rely on the drying method to reduce moisture and break the secretory structures containing the essential oil. Studies have indicated that high drying temperatures facilitate lysigenous cell disruption within the parenchyma, resulting in increased essential oil yield (Hannaa et al., 2012; Pirbalouti et al., 2013).
In addition to the drying process, storage conditions also affect essential oil yield (Lisboa et al., 2018). However, studies on these procedures in Eugenia uniflora are scarce. The optimum storage conditions for Surinam cherry leaves is needed to improve essential oil production.
This study aimed to characterize the essential oil yield and composition of Eugenia uniflora leaves dried at different temperatures for different periods of time and after storage in different types of package materials.
MATERIALS AND METHODS
The plant material was collected in Parana State (1,040 m altitude, 25º02'34'' S and 51º31'47'' W), Brazil, in June (2012).
The leaves of ten Surinam cherry trees, with dark red fruits, were selected for diameter at breast height, ranging from 20 to 32 cm. Exsiccates of fresh branches were deposited in the “Integrated Spiritist College Herbarium”, Curitiba, Parana (register HFIE 9.127).
Drying experiment
The drying experiment was carried out in a completely randomised design, comparing the effect of temperature (room temperature of 22°C and dryer temperature of 45°C) for 6, 24, 48, 72 and 96 h, as compared to non-dried leaves, with three replications The fresh leaf samples were homogenised and separated into sub-samples (approximately 4 kg) for each replication. The leaves were air-dried at room temperature and relative humidity (69%) in the absence of direct sunlight incidence. The controlled temperature drying was done at 45°C with forced ventilation (Nova Ética®).
The essential oil was isolated with hydrodistillation in a Clevenger graduated apparatus using 100 g of leaves in 1 L of distilled water, for 4 h, according to the Brazilian Pharmacopoeia recommendations (ANVISA, 2020). The essential oil components were subsequently identified and quantified with gas chromatography-mass spectrometry (60-240°C, 3ºC min-1 ramp) in an Agilent 7890A device with a DB-5 (30 m × 0.25 mm × 0.25 μm) capillary column, operating in a 1:5 flow divider mode. The injector and detector temperatures were 250 and 280°C, respectively. Helium was used as the carrier gas at a flow of 1 m min-1. The essential oil was diluted in hexane to a concentration of 1%, and an aliquot of this solution (1 μL) of each sample was injected. The mass spectra were obtained with electron ionisation at 70 eV with an acquisition rate of 3.15 scans/s, in the range of 40 to 500 Da. The essential oil components were identified by comparing the retention indices of the components with those of a homologous series of n-alkanes (C11-C24) injected into the same column and were calculated according to the Van den Dool and Kratz (1963) equation. The mass spectra were compared with data from the Wiley/NBS Spectrotech, and the linear retention indices were verified according to the literature data (Adams, 2017).
The essential oil yield was determined by calculating the density and the corrected values for dried mass (DM), and the subsample moisture percentage was determined with 10 g of leaves perr repetition.
Storage experiment
To evaluate the influence of storage type and time on the yield of the essential oil, 200 g of fresh leaves were dried to 11% moisture with the Ecodryer method with a controlled air speed and drying temperature (70ºC on the leaves). The experiment design was completely randomized in a 3 × 4 factorial, comparing the package types and four storage periods (60, 120, 180 d and with no storage), with five replications. The package types included polyethylene raffia bags (70 g m-2), polyethylene transparent bags (10 µm) and double kraft bags (200 g m-2), and wrapped in a polyethylene transparent bags (10 µm). The packaged leaves were kept under a wooden "pallet" measuring 1 x 1.2 m, simulating the storage system used in the medicinal and aromatic plant industries.
Prior to storage, 15 essential oil extractions were performed on fresh leaves (controls) containing 53% moisture. A further 15 essential oil extractions were performed on dried leaves, containing 11% moisture. These extractions provided a statistical comparison related to the storage treatments.
Essential oil yield was determined by calculating the density and the corrected values for dried mass (DM). The subsample moisture percentage was determined with 10 g of leaves per repetition.
The treatment variances were tested for homogeneity with Bartlett's test. The averages were compared with Tukey’s test at 5% probability, using the statistical program ASSISTAT version 7.7 (Silva and Azevedo, 2016).
RESULTS AND DISCUSSION
Drying experiment
Essential oil yield
The drying of Surinam cherry leaves resulted in a significant increase in essential oil yield. The highest average was obtained when the leaves were dried at 45°C for 24 h (3.7 µL g-1 DM) and at ambient temperature for 48 h (3.5 µL g-1 DM) (Tab. 1).
Table 1 Average essential oil yield (µL g-1 DM) of Surinam cherry leaves prepared by different drying treatments.
| Time (h) | Drying temperature | |
|---|---|---|
| Ambient (22°C) | 45ºC | |
| 0 | 2.5 aB | 2.0 bB |
| 6 | 2.2 aBC | 2.0 aB |
| 24 | 2.1 bBC | 3.7 aA |
| 48 | 3.5 aA | 1.8 bB |
| 72 | 1.6 aC | 2.0 aB |
| 96 | 1.7 aC | 1.5 aB |
| CV (%) | 11.3 | |
CV (%) = coefficient of variation; DM = dried mass; Averages with the same lowercase letters in the rows and capital letters in the columns are not significantly different according to Tukey’s test (P<0.05).
Previous results for other aromatic plant species are similar to this study, where a high essential oil yield was found under specific drying temperatures. In Lippia citriodora, the highest essential oil yield was obtained at 30°C, with a significant decrease at higher temperatures (Shahhoseini et al., 2013). Pirbalouti et al. (2013), who compared different drying methods for Satureja bachtiarica, found the highest oil yields at 45°C for 48 h.
The changes in essential oil yield of aromatic plants after drying can be related to the anatomy of the storage structures. According to Fiuza et al. (2008), the essential oil in Eugenia uniflora leaves is located in the palisade parenchyma, within secretory cavities or oil channels. Increasing the drying temperature and consequent water removal from the cells may contribute to degradation of the leaf tissue and release of the essential oil from the secretory cavities of the palisade parenchyma.
However, in other species, especially those from the Lamiaceae family, which mainly accumulate essential oil in glandular trichomes, an increase in drying temperature may reduce the essential oil yield, as observed in Thymus vulgaris (Sározi et al., 2013), Melissa officinalis (Argyropoulos and Müller, 2014) and Hyssopus officinalis (Venditti et al., 2015). In these species, an increase in temperature during the drying process drastically reduces the yield of essential oils because of the high volatization (Abdelmageed et al., 2011).
The volatization process can also decrease the essential oil yield when subjected to prolonged drying times (Sellami et al., 2011; Rahimmalek and Goli, 2013). In this experiment, drying over 48 h at room temperature or 24 h at 45°C reduced the essential oil yield at similar levels from those obtained with fresh leaves. According to Argyropoulos and Müller (2014), the decrease in the essential oil yield may be associated with moisture losses in plant material during the drying process. Prolonged drying times in species such as Eugenia uniflora have a high moisture loss, with a consequent increase in the rupture of the storage structures and volatilization reducing essential oil yield.
Essential oil composition
The essential oil samples of leaves dried at room temperature and 45°C had 12 compounds that were identified, with averages of 60 and 67.1% identification, respectively. Sesquiterpenes were the most representative compounds in the analyzed samples (Tab. 2), which agrees with other studies carried out on the species (Gallucci et al., 2010; Rodrigues et al., 2013). According to Stefanello et al. (2011), this class of compounds is responsible for the biological action of essential oils from species of the Myrtaceae family.
Table 2 Average essential oil composition (%) of Surinam cherry leaves with different drying temperatures.
| Compound* | RIe | RIt | Drying temperature | |
|---|---|---|---|---|
| Ambient (22°C) | 45°C | |||
| (E)-β-Ocimene | 1052 | 1044 | - | 2.1 |
| γ-Elemene | 1433 | 1434 | 1.4 | 1.8 |
| Allo-Aromadendrene | 1460 | 1458 | 1.1 | 1.3 |
| Germacrene D | 1481 | 1484 | 3.7 | 4.9 |
| Viridiflorene + Curzerene | 1498 | 1496/ 1499 | 15.5 | 14.6 |
| Germacrene B | 1557 | 1559 | 3.9 | 5.9 |
| Palustrol + ni | 1569 | 1567 | 2.4 | 2.1 |
| Globulol | 1588 | 1590 | 7.7 | 8.8 |
| Viridiflorol + Cubeban-11-ol | 1595 | 1592/ 1595 | 5.8 | 5.0 |
| Epi-α-Muurolol | 1647 | 1640 | 5.3 | 7.5 |
| α-Cadinol | 1661 | 1652 | 7.4 | 8.6 |
| Germacrone + ni | 1694 | 1693 | 5.8 | 4.5 |
| Monoterpene hydrocarbons | - | 2.1 | ||
| Sesquiterpene hydrocarbons | 10.1 | 13.9 | ||
| Oxygenated sesquiterpenes | 49.9 | 51.1 | ||
| Total identified | 60.0 | 67.1 | ||
ni = not identified; RIe = retention index calculated from the compounds retention times with that of a homologous n-alkane series using a DB-5 column; RIt = retention index Adams (2017); * Identified compounds with an average content above 1%.
The major constituents found in the essential oil of the Surinam cherry leaves were viridiflorene+curzerene (15.5% at 19ºC and 14.6% at 45ºC), globulol (7.7% at 19ºC and 8.8% at 45ºC) and α-cadinol (7.4% at 19ºC and 8.6% at 45ºC) (Tab. 2). According to Pripdeevech and Chukeatirote (2010), these constituents have antifungal and antioxidant properties.
The characteristic scent of Eugenia uniflora essential oil used in the cosmetics industry is associated with nine compounds (globulol, α-cadinol, viridiflorol, espatulenol, β-elemene, γ-elemene, germacrone, furanodiene+curzerene, atractylone). The presence of furanodiene+curzerene, γ-elemene and germacrone provides a spicy and woody aroma similar to the fruit, as verified through an olfactory comparative analysis (Melo et al., 2007).
The analysis of the Surinam cherry essential oil composition in this study indicated that the genetic material presented six olfactory components (curzerene, globulol, α-cadinol, germacrene, viridiflorol and γ-elemene), which contributed to the characteristic scent of Surinam cherry, as reported by Melo et al. (2007). According to the Brazilian Pharmacopeia (ANVISA, 2020), curzerene is one the main Surinam cherry essential oil components, which, in this study, co-eluted with viridiflorene under the chromatographic conditions.
In Eugenia uniflora, the curzerene levels may be associated with the genetic diversity and wide geographic distribution. Costa et al. (2010) correlated the differences in essential oil composition of Surinam cherry leaves with the fruit color and identified three chemotypes with broad variation in the curzerene levels. According to the geographic location of Eugenia uniflora, curzerene levels of 19.7% (Ogunwande et al., 2015), 22.4% (Santos et al., 2015) and 47.3% (Rodrigues et al., 2013) have been found in the essential oil of its leaves.
The results (Tab. 2) demonstrated that drying leaves at 45°C increased the monoterpene and sesquiterpene compounds in the Surinam cherry essential oil. A total of 67% of the identified compounds had an increase in the concentrations, with an increase of 11.83% in sesquiterpenes. Similarly, Corrêa et al. (2006) dried different medicinal and aromatic plants and concluded that the ideal drying temperature was determined by the volatile properties of the essential oil of the plant material.
Changes in the essential oil composition of Surinam cherry were also reported by Sellami et al. (2011) in results from drying and temperature conditions and the consequent oxidative process and chemical rearrangements. In the present study, the decrease and/or increase of certain compounds between different drying temperatures (ambient and 45°C) was related to volatilization ratio and/or chemical changes.
Storage experiment
Essential oil yield
The results showed that the essential oil yield was not significantly affected by the material type in the storage packages for the Eugenia uniflora leaves. On the other hand, there was a significant effect from the storage period on the essential oil yield of the dried Surinam cherry leaves (Tab. 3 and Fig. 1). The reduction in the essential oil yield was 32% after 60 d of storage, 41% after 120 d and 76% after 180 d, as compared to the yield of fresh leaves (Tab. 3).
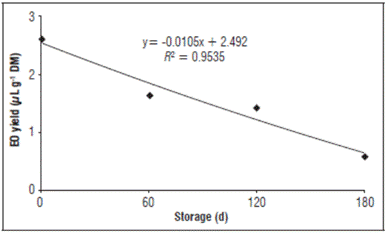
Figure 1 Essential oil (EO) yield of dried Surinam cherry leaves (11.0% moisture) during storage. Storage conditions: relative humidity 59.0-72.0%, temperature 19-29°C; DM = dried mass.
Table 3 Relationship between average essential oil yield from dried Surinam cherry leaves during storage and from fresh (53.0% moisture) and dried (11.0% moisture) leaves without storage.
| Storage (days) | Relationship between EO yield of fresh and dried stored leaves | Relationship between EO yield of 11.0% moisture dried leaves and stored dried leaves |
|---|---|---|
| 0 | 1.0 a | - |
| 60 | 0.7 b | 0.6 a |
| 120 | 0.6 b | 0.5 a |
| 180 | 0.2 c | 0.2 b |
| CV (%) | 23.8 | 31.6 |
CV (%) = coefficient of variation; EO = essential oil. Means with the same lowercase letters in columns are not significantly different according to Tukey’s test (P<0.05).
A reduction in essential oil yield was also observed by other authors, which agree with the data in this study, comparing the effect of storage time of the plant material. Verma et al. (2011) found a reduction of up to 59% in the yield of essential oil extracted from Rosmarinus officinalis after 270 d. According to Dušková et al. (2016), the increase in the storage period of Lavandula angustifolia flowers also decreased the essential oil yield when compared to flowers before storage. The reduction in essential oil yield during storage of plant material could be related to the high volatility of the essential oil components (Zuzarte and Salgueiro, 2015), which have an intrinsic characteristic. The loss in essential oil yield during storage could also be a result of the quality of the plant material. Drying plant material affects the integrity of the structures where the essential oils of Eugenia uniflora leaves are stored because it increases the volatilization of the essential oil components.
CONCLUSION
Drying the Surinam cherry (Eugenia uniflora) leaves increased the essential oil yield after 24 and 48 h at 45°C and at ambient temperature. The levels of monoterpenes and sesquiterpenes also increased when the leaves were dried at 45°C. The major compounds in the essential oil of the evaluated genetic material were curzerene and viridiflorene, which co-eluted under the chromatographic conditions used in this study. The essential oil yield of the Surinam cherry leaves was not influenced by the packaging type during storage. However, storage reduced the essential oil yield of the dried Eugenia uniflora leaves.















