INTRODUCTION
Physalis peruviana (L.) fruits are exotic in terms of their appearance, taste, medicinal properties and nutritional value (Rufato et al., 2008; Puente et al., 2011; Lima et al., 2013; Çakir et al., 2014; El-Beltagi et al., 2019). This fruit is commonly called uchuva (Spanish), cape gooseberry, goldenberry or simply physalis (Muniz et al., 2011; Fischer et al., 2014).
The origin center for P. peruviana is located between the Peruvian and Ecuadorian Andes (Morton and Russel, 1954). It is a perennial and semi-evergreen shrub with indeterminate growth (Fischer and Melgarejo, 2020).
Cultivation in Colombia began in 1985 (Muniz et al., 2014). The cape gooseberry currently plays an important role in the country's export market, following bananas (Fischer et al., 2014). Most of the cape gooseberry supply in Brazil is imported from Colombia, complementing production on small plantations in Santa Catarina, Rio Grande do Sul, Minas Gerais and São Paulo (Rufato et al., 2008; Lima et al., 2009; Muniz et al., 2011; Rodrigues et al., 2012; Betemps et al., 2014).
Cape gooseberry production in Brazil presents an excellent alternative for small and medium producers because of the high added value of fresh fruits (98-150 R$/kg or 25.42-38.90 US$/kg) and ease of cultivation. Production could transform this country from importer to exporter in a short period of time (Rufato et al., 2008).
Success in establishing new crops depends primarily from the propagation material. The cape gooseberry is propagated mainly with seeds (Muniz et al., 2014; Fischer et al., 2014), which is a cheap and efficient method because seeds have high, rapid germination (72.50% at 7 days after sowing (DAS); 82.50% at 21 DAS) (Melo et al., 2015). However, obtaining seedlings takes a long time, around 2 months (Miranda, 2005), which increases the cost of seedlings for producers and nurseries.
The production of quality seedlings depends on several factors associated with the substrate composition (porosity, air and water retention, and electrical conductivity), nutritional supplementation (macronutrients and micronutrients), containers (volume, shape, cost), irrigation (frequency, quantity and water quality) and environment (temperature, humidity, photoperiod, radiation) (Villa et al., 2018; Reyes et al., 2019; Roveda-Hoyos and Moreno-Fonseca, 2019; Marchioretto et al., 2020). There are several substrate recommendations for cape gooseberries: washed sand, charred rice husks, turbid, Brazilian coco peat and mycorrhizae are the more common ones (Miranda, 2005; Díaz et al., 2010). Organic sources, such as manure and vermicompost, are excellent alternatives from the environmental and phytotechnical points of view (Inácio and Miller, 2009).
The objective of this study was to determine the effect of commercial substrates and organic sources on the production of cape gooseberry seedlings.
MATERIALS AND METHODS
The cape gooseberry seeds came from plants in the germplasm bank of UDESC (Universidade Estadual de Santa Catarina), at Lages, State of Santa Catarina (Brazil). The seeds were then kept in a cold chamber (5°C).
The design was completely randomized with a 2×3 factorial scheme using two commercial substrates (Carolina® - Carolina Soil Company, Santa Cruz do Sul/Rio Grande do Sul/Brazil; Bioplant® - Bioplant Agrícola Company, Nova Ponte - Minas Gerais/Brazil) and three organic sources (none, vermicompost enriched with yoorin thermophosphate (Guimarães et al., 2017) and poultry manure).
The physical and chemical characterization of the organic compounds is shown in table 1.
Table 1 Physical and chemical characterization of organic sources for substrates in the production of cape gooseberry seedlings.
| Parameter | Poultry manure | Vermicompost |
|---|---|---|
| N (g kg-1) | 28 | 8.2 |
| P2O5 (g kg-1) | 65 | 76 |
| K2O (g kg-1) | 30 | 5.5 |
| Ca (g kg-1) | 105 | 82 |
| Mg (g kg-1) | 10 | 37 |
| Sulfur (g kg-1) | 4 | 2.2 |
| Copper (mg kg-1) | 90 | 310 |
| Iron (mg kg-1) | 13200 | 88500 |
| Manganese (mg kg-1) | 800 | 360 |
| Zinc (mg kg-1) | 500 | 2500 |
| Boron (mg kg-1) | 480 | 400 |
| Organic matter (g kg-1) | 415 | 120 |
| Humidity (g kg-1) | 95 | 280 |
| Mineral material (g kg-1) | 490 | 600 |
| pH | 8.6 | 6.87 |
| C/N Ratio | 9.5 | 11.8 |
| Organic matter (%Dry Matter) | 45.9 | 16.7 |
| Electrical conductivity (mS cm-1) | 8.01 | 2.78 |
| Apparent density (g mL-1) | 0.54 | 1.29 |
| Real density (g mL-1) | 1.85 | 1.9 |
| Porosity (%) | 70.8 | 32.1 |
The treatments with commercial and organic sources used a 9/1 ratio (90% commercial substrate and 10% organic source). The commercial and composite substrates underwent a physicochemical analysis to determine N, P2O5 (colorimetry with molybdate), K2O (flame photometry), Ca and Mg (atomic absorption), S (turbidimetry), Cu, Fe, Zn and Mn (atomic absorption), B (colorimetry with azomethine), organic matter, moisture, mineral matter and pH (potentiometry), C/N, organic matter (% dry matter), electrical conductivity, real density and apparent density.
The seeds were sown in Styrofoam trays with 128 cells (40 cm³/cell) filled with different combinations of commercial substrate and organic sources. Two seeds were placed in each cell. Six replicates of 36 seeds were used.
The trays were kept in a protected environment (greenhouse). A thermo-hygrometer data logger (AK172) was installed one and a half meters above the greenhouse floor. Daily climatic data, namely temperature (minimum, maximum, average) and relative humidity (mean), were collected. The thermal amplitude and the accumulated degree-days (ADD) were calculated. The ADD values were calculated with an equation adapted from McMaster and Wilhelm (1997) (Eq. 1)
where, ADD is the accumulated degree-days or accumulated thermal sum in °C, Ti is the average daily temperature in °C, Bt is the base temperature (6.3 ° C) (Salazar et al., 2008), n is the number of days and i is the day.
Seedling emergence was evaluated at 5, 8, 12, 16 and 21 DAS to calculate the emergence rate (Maguire, 1962). Seedling thinning was carried out during the emergence evaluation period, leaving only 1 seedling per cell.
A seedling growth analysis was performed at 47 DAS. The following variables were obtained: shoot height (cm), collecting diameter (mm), number of leaves, chlorophyll content (Falker Chlorophyll Index, FCI), total dry weight (g), shoot dry weight (g), root dry weight (g), shoot/height ratio and Dickson quality index (Dickson et al. 1960).
Three seedlings were sampled in each repetition. The shoot height and the hypocotyl diameter were determined using a graduated ruler and a pachymeter, respectively. The chlorophyll content was estimated using a CFL1030 meter (Falker). The seedlings were conditioned in paper bags and subjected to the greenhouse method (60°C, 72 h) (Benincasa, 2003) to determine the dry weight of the shoots, roots and total dry weight.
The F Test was used to determine significance in the effects of the treatments, with the mean "organic sources" and the interactions between the "organic sources" and "commercial substrates" compared with the Tukey test. A correlation analysis (Pearson) was carried out between the phytotechnical attributes (emergence and biometric) and the physicochemical composition of the substrates.
RESULTS AND DISCUSSION
Emergence - The cape gooseberry seedling emergence began 8 DAS. This period was shorter than most seedling production systems in Colombia, where emergence onset occurs from 10 to 15 d. Precocity is associated with thermal conditions (Muniz et al., 2014). There were high diurnal temperatures (on average 28°C) and low nocturnal temperatures (on average 21.7°C), with a mean thermal amplitude of 16.7°C up to 8 DAS.
Adding poultry manure to the commercial substrates had deleterious effects on the precocity and emergence viability (Fig. 1). Similar results have also been reported for other species, namely Cassia siamea, Dolonix regia, Leucaena leucocephala, Mimosa caesalpiniafolia, Enterolobium cotortosilicum (De Lucena et al., 2004), Solanum lycopersicon, Cucurbita pepo, Capsicum annum (Medina et al., 2009), Tamarindus indica (Queiróz et al., 2011), Passiflora edulis (Brugnara, 2014), Lepidium sativus (Rombola et al., 2015) and Zea mays (Vozhdayev et al., 2015).
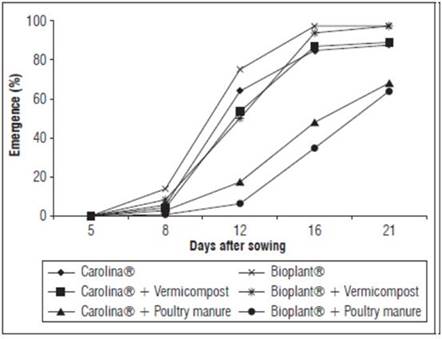
Figure 1 Evolution of cape gooseberry seedling emergence with different commercial substrates and organic sources.
The substrates with poultry manure had four crucial characteristics that contributed to a low seedling-emergence: high nitrogen, potassium and sulfur levels and high electrical conductivity (values above 2.0 mS cm-1) (Tab. 2). These characteristics, along with the C/N ratio, were negatively correlated with the emergence percentage and emergence rate (Tab. 3).
Table 2 Physical and chemical composition of the substrates composed of commercial products and organic sources used in cape gooseberry seedling production. Car. - Carolina®; Bio. - Bioplant®; Vermi. - Vermicompost; Poultry M. - Poultry manure.
| Car. | Bio. | Car. + Vermi. | Car. + Poultry M. | Bio. + Vermi. | Bio. + Poultry M. | |
|---|---|---|---|---|---|---|
| N (g kg-1) | 9.2 | 9 | 8 | 14 | 8.2 | 15 |
| P2O5 (g kg-1) | 13.5 | 10.9 | 38 | 35 | 47 | 36 |
| K2O (g kg-1) | 4 | 4 | 6 | 14 | 6.5 | 12.5 |
| Ca (g kg-1) | 12.7 | 8.6 | 38 | 55 | 36.5 | 35.4 |
| Mg (g kg-1) | 61 | 11 | 40 | 42 | 16.3 | 11.4 |
| Sulfur (g kg-1) | 2.6 | 2 | 2.8 | 3.5 | 1.7 | 3 |
| Copper (mg kg-1) | 10 | 20 | 160 | 30 | 120 | 240 |
| Iron (mg kg-1) | 29,290 | 22,100 | 55,000 | 23,400 | 43,200 | 22,200 |
| Manganese (mg kg-1) | 260 | 430 | 1800 | 380 | 1500 | 600 |
| Zinc (mg kg-1) | 70 | 70 | 1500 | 200 | 900 | 250 |
| Boron (mg kg-1) | 500 | 600 | 500 | 450 | 600 | 550 |
| Organic matter (g kg-1) | 290 | 350 | 220 | 290 | 280 | 315 |
| Humidity (g kg-1) | 410 | 450 | 360 | 330 | 440 | 425 |
| Mineral material (g kg-1) | 300 | 200 | 420 | 380 | 280 | 260 |
| pH | 5.6 | 6.02 | 6.19 | 7.01 | 6.76 | 6.91 |
| C/N ratio | 31 | 41 | 24.9 | 17.9 | 35.4 | 21.2 |
| Organic matter (%Dry matter) | 49.2 | 63.6 | 34.4 | 43.3 | 50 | 54.8 |
| Electrical conductivity (mS cm-1) | 1.72 | 1.09 | 1.78 | 2.67 | 1.81 | 2.91 |
| Apparent density (g mL-1) | 0.18 | 0.37 | 0.33 | 0.25 | 0.49 | 0.4 |
| Real density (g mL-1) | 0.57 | 1.1 | 1 | 0.73 | 1.35 | 1.3 |
| Porosity (%) | 68.4 | 66.4 | 67 | 65.7 | 63.7 | 69.2 |
Table 3. Correlation matrix between emergence attributes and physicochemical composition of substrates used to produce cape gooseberry seedlings.
| 8E | 12E | 16E | 21E | ESI | N | K2O | S | C/N | EC | |
|---|---|---|---|---|---|---|---|---|---|---|
| 8E | 1 | |||||||||
| 12E | 0.804 | 1 | ||||||||
| 16E | 0.836 | 0.967 | 1 | |||||||
| 21E | 0.777 | 0.968 | 0.983 | 1 | ||||||
| ESI | 0.860 | 0.993 | 0.964 | 0.954 | 1 | |||||
| N | -0.650 | -0.972* | -0.916* | -0.933* | -0.939* | 1 | ||||
| K2O | -0.717 | -0.921* | -0.844* | -0.813* | -0.929* | 0.906* | 1 | |||
| S | -0.790 | -0.790 | -0.883* | -0.785 | -0.800 | 0.726 | 0.736 | 1 | ||
| C/N | 0.935* | 0.840* | 0.883* | 0.793 | 0.884* | -0.724 | -0.833 | -0.924 | 1 | |
| EC | -0.893* | -0.951* | -0.892* | -0.872* | -0.976* | 0.878 | 0.930 | 0.738 | -0.887 | 1 |
8E - 8E - Emergence 8 days after sowing (DAS); 12E - 12 DAS; 16E - 16 DAS; 21E - 21 DAS; ESI - Emergence speed index; N - Nitrogen (g kg-1); K2O - Potassium (g kg-1); S - Sulfur (g kg-1); C/N - C/N ratio; EC - Electrical conductivity (mS cm-1). * - significant (P<0.05).
The high levels of nitrogen, potassium and sulfur caused an ionic imbalance, culminating in saline and osmotic stress (Parida and Das, 2005; Chan et al., 2008; Libra et al., 2011) with consequent root toxicity. In addition, water absorption must have been altered because of the osmotic potential change (Mokhele et al., 2012; Liu et al., 2014). Therefore, membrane functionality and enzymatic complexes were compromised, causing deficient and/or delayed root protrusion and aerial emission.
Biometric attributes of cape gooseberry seedlings
An interaction was observed between the factors "commercial substrates" and "organic sources" for the variables emergence speed index, number of leaves, total dry weight, root dry weight, shoot dry weight and Dickson quality index (Tab. 4). The other variables were considered relative to the means of each factor.
Table 4. Seedling emergence at 21 days after sowing (E21), emergence speed index (ESI), height (H), hypocotyl diameter (HD), diameter/shoot height ratio (DHR), number of leaves (NL), total dry weight (TDW), root dry weight (RDW), shoot dry weight (SDW), chlorophyll content (CHL) and Dickson quality index (DQI) of cape gooseberry seedlings produced with different commercial substrates and organic sources.
| Treatment | E21 (%) | ESI | H (cm/ plant) | HD (mm/ plant) | DHR | NL (leaves/ plant) | CHL (FCI) | TDW (mg/ plant) | RDW (mg/ plant) | SDW (mg/ plant) | DQI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate (Subs) | |||||||||||
| Carolina® | 92.12 | 7.57 | 7.38 | 2.24 | 3.27 | 7.52 | 29.77 | 0.26 | 0.05 | 0.20 | 0.03 |
| Bioplant® | 92.59 | 7.84 | 4.41 | 1.96 | 2.20 | 6.68 | 25.38 | 0.16 | 0.03 | 0.13 | 0.02 |
| Test F | 0.04 NS | 1.53 NS | 56.09** | 14.14** | 74.60** | 9.93** | 15.54** | 23.27** | 12.78** | 15.61** | 4.20** |
| Organic source (OS) | |||||||||||
| Control | 95.83 a | 9.07 a | 4.66 b | 1.93 b | 2.34 b | 6.38 b | 25.82 b | 0.17 b | 0.04 b | 0.12 b | 0.03 ab |
| Vermicompost | 97.22 a | 8.58 a | 6.15 a | 2.25 a | 2.70 b | 7.33 a | 27.00 ab | 0.20 ab | 0.06 a | 0.14 b | 0.04 a |
| Poultry manure | 84.02 b | 5.47 b | 6.86 a | 2.13 ab | 3.16 a | 7.59 a | 29.90 a | 0.27 a | 0.03 b | 0.23 a | 0.02 b |
| Test F | 15.28** | 100.33** | 10.69** | 6.57** | 14.48** | 6.92** | 4.72* | 7.02** | 11.12** | 12.82** | 6.15** |
| Test F (Subs×OS) | 3.13NS | 7.30** | 1.04NS | 1.92NS | 3.18 ns | 4.44* | 2.15NS | 9.08** | 7.76** | 10.41** | 7.77** |
* significant (P<0.05); ** significant (P<0.01); NS non-significant. Means followed by the same letter do not differ according to Tukey’s test (P>0.05).
The commercial substrate Carolina® enabled cape gooseberry seedling growth with higher seedling biometric quality than with the substrate Bioplant® (Tab. 4 and 5). The differences were 67% (height), 14% (hypocotyl diameter), 48% (height/diameter ratio), 37% (number of leaves), 17% (chlorophyll content), 385% (total dry weight), 700% (root dry weight), 400% (shoot dry weight) and 400% (Dickson quality index).
Table 5. Emergence speed index (ESI), number of leaves (NL), total dry weight (TDW), dry matter mass (RDW), shoot dry weight (SDW) and Dickson quality index (DQI) of cape gooseberry seedlings produced with different commercial substrates and organic sources.
| Control | Vermicompost | Poultry manure | |
|---|---|---|---|
| ESI | |||
| Carolina® | 8.51 bA | 8.26 aA | 5.92 aB |
| Bioplant® | 9.62 aA | 8.89 aA | 5.02 bB |
| NL | |||
| Carolina® | 7.38 aA | 7.56 aA | 7.63 aA |
| Bioplant® | 5.38 bB | 7.11 aA | 7.55 aA |
| TDW | |||
| Carolina® | 0.27 aAB | 0.20 aB | 0.32 aA |
| Bioplant® | 0.07 bB | 0.21 aA | 0.21 bA |
| RDW | |||
| Carolina® | 0.07 aA | 0.07 aA | 0.03 aB |
| Bioplant® | 0.01 bB | 0.06 aA | 0.03 aB |
| SDW | |||
| Carolina® | 0.20 aA | 0.12 aB | 0.29 aA |
| Bioplant® | 0.05 bB | 0.17 aA | 0.17 bA |
| DQI | |||
| Carolina® | 0.04 aA | 0.04 aA | 0.02 aB |
| Bioplant® | 0.01 bB | 0.04 aA | 0.02 aAB |
Means followed by the same lowercase letter in a column and the same uppercase letter in a row do not differ according to Tukey's test (P>0.05).
The superiority of the Carolina® substrate was attributed to its higher nutritional content (nitrogen, phosphorus, calcium, magnesium and sulfur) and balance of physical characteristics (apparent density and actual density) (Tab. 2). Cape gooseberry seeds are very small and quickly deplete their reserves, so the substrate must provide necessary nutrients and satisfactory aeration conditions for seedling growth and development (Melo et al., 2015). The ideal substrate provides the conditions needed rapid and uniform emergence and vigorous, proportional development (Ferreira et al., 2009). The Bioplant® substrate acts more like an emergence conditioner. Other studies have reported a lack of adequate physical and chemical conditions for seedling production in other species when fertilization supplementation is primordial (Almeida et al., 2014). Dutra et al. (2012) reported that Bioplant® led to the formation of copaiba seedlings (Copaifera langsdorffii) that were deficit in the accumulation of leaf and total dry weight. Ferreira et al. (2009) described this substrate as causing asymmetry between the shoot and root dry weights in cupuaçu seedlings (Theobroma grandiflorum).
The fundamental physical and chemical attributes for the growth of cape gooseberry seedlings are: moisture, mineral matter, C/N ratio, organic matter and boron and sulfur contents (Tab. 6). Only these attributes presented a correlation with the biometric attributes.
Table 6. Correlation between emergence speed and biometric attributes and the physical and chemical composition of composite substrates in cape gooseberry seedling production.
| ESI | SH | HD | DHR | NL | CHLO | TDW | SDW | DQI | S | B | OM | Hum | MM | C/N | OM (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESI | 1 | |||||||||||||||
| SH | -0.511 | 1 | ||||||||||||||
| HD | -0.311 | 0.856* | 1 | |||||||||||||
| DHR | -0.553 | 0.983* | 0.758 | 1 | ||||||||||||
| NL | -0.634 | 0.871* | 0.857* | 0.852* | 1 | |||||||||||
| CHLO | -0.674 | 0.875* | 0.746 | 0.844* | 0.745 | 1 | ||||||||||
| TDW | -0.554 | 0.873* | 0.620 | 0.932* | 0.858* | 0.643 | 1 | |||||||||
| SDW | -0.616 | 0.761 | 0.421 | 0.852* | 0.740 | 0.536 | 0.960* | 1 | ||||||||
| DQI | 0.264 | 0.425 | 0.689 | 0.353 | 0.558 | 0.119 | 0.400 | 0.181 | 1 | |||||||
| S | -0.800 | 0.781 | 0.493 | 0.806 | 0.621 | 0.930* | 0.652 | 0.642 | -0.182 | 1 | ||||||
| B | 0.484 | -0.925* | -0.648 | -0.943* | -0.670 | -0.891* | -0.792 | -0.714 | -0.166* | -0.875 | 1 | |||||
| OM | -0.007 | -0.701 | -0.940* | -0.575 | -0.667 | -0.521 | -0.428 | -0.214 | -0.785 | -0.219 | 0.468 | 1 | ||||
| Hum | 0.434 | -0.908* | -0.700 | -0.885* | -0.618 | -0.841* | -0.689 | -0.633 | -0.135 | -0.810 | 0.921* | 0.589 | 1 | |||
| MM | -0.252 | 0.908* | 0.913* | 0.828* | 0.719 | 0.773 | 0.633 | 0.487 | 0.497 | 0.593 | -0.792 | -0.880* | -0.903* | 1 | ||
| C/N | 0.884* | -0.837* | -0.664 | -0.836* | -0.822* | -0.908* | -0.737 | -0.712 | -0.021 | -0.924* | 0.784 | 0.396 | 0.777 | -0.669 | 1 | |
| OM (%) | 0.176 | -0.868* | -0.958* | -0.772 | -0.747 | -0.700 | -0.603 | -0.426 | -0.644 | -0.469 | 0.696 | 0.953* | 0.804 | -0.981* | 0.595 | 1 |
* - significant (P<0.05). ESI - Emergence speed index; SH - Shoot height; HD - Hypocotyl diameter ; DHR - Diameter/height ratio; NL - Number of leaves; CHLO - Chlorophyll; TDW - Total dry weight; SDW - Shoot dry weight; DQI - Dickson quality index; S - Sulfur; B - Boron; OM - Organic matter; Hum - Humidity; MM - Mineral matter; C/N - C/N ratio; OM (%) - Organic matter.
The boron content and humidity were negatively correlated with the shoot height, height-diameter ratio, and chlorophyll content (Tab. 6). Cape gooseberry seedlings may not tolerant inadequate boron levels in substrates. This tolerance depends on the transport speed from the roots to the shoot (Salvador et al., 2003). No symptoms of boron toxicity were observed in the cape gooseberry seedlings. Other studies should be conducted to determine the nutritional requirements for boron and other nutrients in the seedling production phase.
The C/N ratio had the opposite effect on the height, diameter/height ratio, number of leaves and chlorophyll content (Tab. 6). A substrate with a high C/N ratio has low degradability, low nutrient availability and, depending on the seedling production period, deficient nitrogen (Maeda et al., 2007). Nitrogen is a key component of several molecules that are essential for the primary and secondary metabolism of plants (proteins, nucleic acids, hormones, chlorophyll and vitamins). Therefore, an unbalanced supply of this nutrient impacts several biosynthetic pathways and consequently the growth and allocation of dry weight (Xu et al., 2012).
The seedlings were ready for transplant at 47 DAS, when over four true leaves had fully expanded (Tab. 5). The ADD was 1,054°C. In Colombia, the average period for obtaining transplanted seedlings is 60 d (Angulo, 2005). In addition to the delayed early emergence (10-15 DAS), seeds are sown in trays, and seedlings are transplanted to 1 L plastic bags 15 days after emergence, where they stay for another 30 d (Miranda, 2005). Transplant suitability encompasses criteria related to shoot height (15-20 cm), hypocotyl diameter (0.5 cm) and number of leaves (3-4) (Miranda, 2005; Angulo, 2005). The seedlings produced only with the Bioplant® substrate had on average of 5.38 leaves at 47 DAS (Tab. 5) but they were not suitable for transplant because of a low height, minimum accumulation of dry weight and low quality, as indicated by the Dickson index (Tab. 4). Seedlings may not have to reach a height of 15 to 20 cm because several other Solanaceae (tomato, eggplant, pepper) species are successfully transplanted with lower heights. Therefore, the transplanting criteria need to be better established, based on a combination of attributes related to climatic conditions (degree-days), biometric attributes and local productive systems. The production of cape gooseberry seedlings with the Carolina® substrate did not require supplementation with organic sources. An alternative for supplementation in the Bioplant® substrate is vermicompost enriched with yoorin thermophosphate, a material with excellent chemical and physical attributes (Tab. 1). The use of poultry manure must be carefully mediated because of deleterious effects on emergence.
CONCLUSION
Carolina® is a suitable substrate for the production of cape gooseberry seedlings without the need for organic-source supplementation. Bioplant® acts more like an emergence conditioner that requires supplementation with organic source (preferably with vermicompost).
Seedlings that are suitable for transplant can be obtained at 47 DAS.















