INTRODUCTION
Benincasa hispida, known as Chinese cucumber, is a cucurbitaceae grown mainly in South and East Asia, where it grows as a climbing or creeping plant, with hairy stems, triangular leaves in alternate arrangement, and small forked strands (Mandana et al., 2012; Ekeke et al., 2019). Benincasa is a monotypic genus with a single cultivated species [Benincasa hispida (Thunb.) Cogn, 2n = 2x = 24] (Pradhan et al., 2020). The importance of the species is given by its cultural and collective value in food, nutrition, biomedicine, traditional medicine and pharmacological industry, beverages, charcuterie, delicatessen, handicrafts, cultural and religious traditions, among others (Chomicki et al., 2020).
The fruits can be cylindrical, oblong or rounded, depending on the cultivar; provided with a thick wax in the epidermis when they mature and reach a weight of up to 13.67 kg (Pandey et al., 2015). The seeds, located in the center of the fruit, are oval-elliptical, flattened, smooth and colored light yellow, they measure between 1.0 and 1.5 cm long and 0.5 to 0.8 cm wide, however, their dimensions vary with the shape of the fruit (Pradhan et al., 2020).
In Colombia, it has been little investigated and therefore different aspects such as morphological and biometric variability of the seeds are unknown. Biometric studies of fruits, seeds and their associated traits have made it possible to understand the genetic variability of a population (Ekeke et al., 2019), to base the knowledge related to the propagation and germination of seeds (Steiner et al., 2019), and serve to understand the yield, quality and postharvest handling of fruits and seeds (Cañadas-López et al., 2018). On the other hand, the analyses of the morphometry, viability and germination of the seed are key in the production and commercialization of seeds with excellent physiological quality and in the knowledge of the exogenous stimuli that can impair the embryo (Hartmann-Filho et al., 2016).
The tetrazolium test is important for assessing seed viability and its application depends on the protocol of each species. Therefore, in the standardization of this test, seed preparation, tetrazolium solution concentration and staining time are important. These standardization efforts are significant because they directly influence the intensity and uniformity of the seed staining process and, therefore, the evaluation and interpretation of the results compared to conventional germination tests (Souza et al., 2010).
The objective of the study was to evaluate the morphometry, viability and germination of B. hispida seeds, to generate basic information for the conservation, sustainable use and quality control of the seed.
MATERIALS AND METHODS
The study was carried out between August/2016 and July/2017 in the laboratory of Genetics and Plant Breeding of the University of Cordoba (Monteria-Colombia).
The seeds used in the study consisted of 50 fresh, complete, healthy, mature and free pollination fruits taken randomly at harvest in a batch of 0.5 ha of B. hispida. From each fruit 50 seeds were taken at random, to form a balanced compound, which was stored in a cold room ± 5ºC (Espitia et al., 2017; Ekeke et al., 2019).
The morphometric characteristics were estimated in a sample of 100 seeds taken randomly from the balanced compound originating from 50 free pollination fruits.
Eight biometric characteristics of the seeds were estimated: maximum seed width (AS), maximum length (LS) and maximum thickness (GS), measured in cm using a Spictools® vernier. Likewise, the weight of one seed (P1S) and the weight of one hundred seeds (P100S) in g were estimated, in a precision balance 0.01 g, number of seeds per kilogram (NSK), estimated by counting the number of seeds in five samples of 100 seeds, then the average was taken to kg by the respective expansion factor, the volume of a seed (V1S) was estimated in mL as the average increase in volume that is generated in a test tube with a known distilled water volume when introducing a sample of 100 seeds taken at random, and the density of a seed (D1S) was estimated from the ratio (P1S/V1S).
In the external and internal morphological description, the essential anatomical parts of 10 complete and healthy seeds were identified and described, following the methodology proposed by Niembro (1988). The seeds were hydro-pressed in distilled water for 12 h at a constant temperature of 27°C. Subsequently, the seed coat was separated and the internal parts of the seed were fully exposed for description and performance of the tetrazolium test (MAPAB, 2009).
The viability of the seeds studied through topological patterns is used to compare and characterize the staining of the seeds through three categories proposed by Rao et al. (2007) and MAPAB (2009): Category 1: Viable seeds that have fully stained embryo and endosperm, sometimes with superficial necrosis in the middle of the endosperm in the regions furthest from the embryo and behind the radicle. Category 2: Non-viable seeds that present the embryo and endosperm unstained, or at least one of the two is stained, the embryo and radicle apex may present acute necrosis or there is serious damage in more than half of the essential parts of the seeds. Category 3: Doubtful partially stained seeds, where more than half are unstained, have healthy essential parts and can produce normal or abnormal seedlings, depending on the intensity and pattern of the staining.
For the characterization of topological patterns, preconditioned and cut seeds were immersed in a 1% solution of 2,3,5-triphenyl tetrazolium chloride, with a staining time of 2 h (Pinto et al., 2009; MAPAB, 2009). Subsequently, the seeds were introduced into a DIES® oven at a temperature of 40ºC (Espitia et al., 2017), then they were washed three times with distilled water to remove the excess of the dye and the staining of the tissues was observed with the help of a Vista Vision® stereoscope.
For the variation in the seed staining pattern, six treatments were evaluated, originating from the combination of three concentrations of tetrazolium (0.5, 1.0, and 1.5%) with two immersion times (2 and 3 h) of the seeds in the solution with 50 seeds each. The staining efficiency of the embryos was considered, based on the intensity and uniformity of the color (Pinto et al., 2009; MAPAB, 2009) and photographs were taken with a digital camera. For the percentage of viable seeds in this test, the total number of seeds from the “Viable” category (V) was taken, plus half of the seeds from the “Doubtful” category (D), that is, the category (V + 0.5D) (Espitia et al., 2017).
Germination tests and effect of tetrazolium
The estimation of the viability of the seeds by means of the conventional germination test was carried out in a covered mesh house covered with black polypropylene, anti-aphid mesh, 33% shading, average temperature of 29°C and relative humidity of 70%. Four replications of 50 seeds were used, which were sown at a distance of 5 cm between rows and 3 cm between seeds, in plastic trays with a substrate of 50% quartzite sand and 50% clay, both disinfected with hot water (100oC). The sowing depth was 2/3 of the size of the seed, with the part where the radicle emerges downwards (Espitia et al., 2017). Two daily irrigations were carried out at 10:00 AM and at 4:00 PM over 20 d. Germination was assessed daily by recording the number of healthy and normal seedlings emerging over 20 d. Germination was considered when the cotyledons were raised from the substrate level.
The following were evaluated: days at the beginning of germination (DIG), days during germination (DDG), days at the end of germination (DFG), the percentage of germination (PG); the germination speed index (IVG) was calculated using the formula recommended by Maguire (1962) Eq. 1:
where: P1,P2, P3,…, Pn was number of normal, germinated and complete seedlings in the first, second, third and last count of the evaluation, and T1, T2, T3,…, Tn = time in days for each germination.
The mean daily germination (GDM) was considered as the ratio between the cumulative percentage of germinated seeds at the end of the trial and the number of days elapsed from sowing to the end of the trial, the peak value (VP) as the maximum GDM reached in the test and, the germination value (VG) corresponded to the product of the GDM by the VP (Czabator, 1962).
To estimate the effects of tetrazolium treatments, an experiment was carried out under a completely random design, with six treatments and four repetitions of 50 seeds each; the six treatments corresponded to the combinations of three tetrazolium concentrations (0.5, 1.0 and 1.5%) and two staining times (2 and 3 h).
For the evaluation of the characteristics of the seeds, descriptive statistics were carried out and 95% probability confidence intervals were estimated. To estimate the effects of the tetrazolium treatments, a normality (Lilliefors) and homogeneity of variance (Bartllet), analysis of variance and the Tukey's multiple range test were performed at 5% probability. The free access computer program, Windows GENES version V.2014.6.1 (Cruz, 2016) was used. The physiological parameters of germination were calculated using Microsoft Excel v. 2013.
Evaluation of seed biometrics
Descriptive statistics and interval estimates of seed characteristics (Tab. 1) show lower dispersion in LS, GS, AS, P100S, and NSK, but are within the values reported by Ekeke et al. (2019).
Table 1. Descriptive statistics for eight biometric characteristics of B. hispida seeds in the department of Córdoba (Colombia).
| Variable | Mean1 | Min. | Max. | Var. | CV (%) | Standard deviation | IC (95%)2 | |
|---|---|---|---|---|---|---|---|---|
| LI | LS | |||||||
| LS (cm) | 1.03 | 0.93 | 1.20 | 0.0022 | 4.61 | 0.047 | 1.021 | 1.049 |
| GS (cm) | 0.16 | 0.15 | 0.19 | 0.000049 | 4.45 | 0.007 | 0.167 | 0.172 |
| AS (cm) | 0.57 | 0.51 | 0.70 | 0.001936 | 7.66 | 0.044 | 0.563 | 0.589 |
| P1S (g) | 0.03 | 0.03 | 0.05 | 0.000016 | 11.71 | 0.004 | 0.037 | 0.04 |
| P100S (g) | 3.78 | 3.02 | 4.67 | 0.1218 | 9.24 | 0.349 | 3.679 | 3.885 |
| NSK (#) | 26,632 | 21,413 | 33,113 | 5867,925 | 9.09 | 2,422 | 25,895 | 27,317 |
| V1S (mL) | 0.05 | 0.03 | 0.10 | 0.0002 | 26.27 | 0.013 | 0.046 | 0.054 |
| D1S (g mL-1) | 0.80 | 0.40 | 1.29 | 0.0259 | 20.17 | 0.161 | 0.752 | 0.846 |
1: Average of 50 data; Min. = Minimum value, Max. = Maximum value; Var.: Variance; CV: Coefficient of variation; 2: IC (95%): Confidence interval at P≤0.05; LI: Lower limit; LS: Upper limit; LS: Seed length; GS: Thickness of the seed; AS: Seed width; P1S: Weight of a seed; P100S: Weight of 100 seeds; NSKG: Number of seeds/kg; V1S: Volume of a seed; D1S: Density of a seed.
The variability detected in the characters LS, GS, AS, NSK and P100S, indicates high phenotypic homogeneity (homogeneous-homozygous) of the seed, a fact that reduces the possibility of successful selection and genetic advancement (Shafique et al., 2016). This is possibly due to the Founder effect and this forces the introgression of new genes that lead to the generation of genetic variability, when the objective is to improve the characteristics of the seeds (Shafique et al., 2016; Dutta et al., 2018).
Description of the external and internal morphology of the seed
The seeds of B. hispida are oval-elliptical, flattened, smooth, the testa can vary between cream-white and pale yellow, agreeing with that reported by Ekeke et al. (2019). Inside, the embryo is axial, linear, white or cream in color and is wrapped in a thin membrane which makes it waterproof, which resembles a placentation (Agbagwa and Ndukwu, 2004). It has two cotyledons that are flat, thin, uniform and whitish, while the radicle is short (Fig. 1), which is consistent with previous studies by Ekeke et al. (2019).
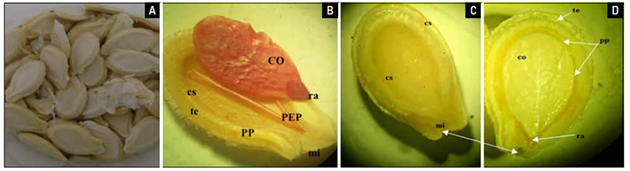
Figure 1. Anatomical characteristics of the Benincasa hispida seed (Thunb.) Cogn. A) Superficial and external view of the seed; B), C) and D) Internal view of the seed: te: Testa; PP: Waterproofing protective film; cs: seminal cover; mi: micropyle; co: cotyledons, ra: radicle.
The external and internal morphological characters of the B. hispida seed are related and similar to the seeds of cucurbits and their biometric values are a function of genetic and environmental effects and are very similar to those reported by Delgado et al. (2014), for Cucurbita moschata, since they are piriform, mostly cream in color, and with a thin, smooth to rough edge.
Determination of topological patterns of sedes
Four classes or staining patterns were differentiated and identified in the seeds of B. hispida, (Tab. 2) and are described as follows: Class 1. Viable seeds, with intense, total and uniform staining in all internal parts of the seed (cotyledons, embryonic axis, radicle and micropyle); Class 2. Viable seeds with staining in more than 80% of the radicle and embryo; Class 3. Doubtful seeds with red to pink staining up to 50% of cotyledons, embryonic axis and radicle and Class 4. Inviable seeds without staining. These variations in the intensity of the staining of the internal parts of the seeds are in agreement with Macedo-Sousa et al. (2017), and originate from the interaction of living cells with the colorless solution of 2,3,5-triphenyl tetrazolium chloride, this allows the release of hydrogen ions from dehydrogenase enzymes and, by hydrogenation, a red, stable and non-diffusible substance called Triphenylformazan is produced in living cells, indicates the respiratory activity of the mitochondria and delimits the tissue with deficient physiological activity by its color (MAPAB, 2009; Macedo-Sousa et al., 2017; Hidayat and Ridhawati, 2020). Therefore, the intense red color in the embryos is a positive indicator of the viability and high vigor of the seeds (Class 1 and 2).
Table 2. Topological patterns interpreted in the tetrazolium test in B. hispida seeds. (Thunb.) Cogn.
Faintly colored or pinkish seeds in some parts of the embryo (Class 3), indicate that the cells have a decreased respiratory activity (MAPAB, 2009; Hidayat and Ridhawati, 2020) and consequently less activity of dehydrogenase enzymes, this type of staining can result in abnormal plants (Macedo-Sousa et al., 2017). Finally, the parts that do not present coloration (Class 4), can be classified as non-viable with the absence of metabolic activity in the seed, necessary for the production of Triphenylformazan (Macedo-Sousa et al., 2017).
Evaluation of seed viability
Table 3 shows the analysis of variance and the means comparison tests for viability and germination of the seeds, in which differences (P<0.05) were observed between the treatments, only for the percentage of viable seeds (V) with P>F: 0.027* and doubtful (D) with P>F: 0.007**, not so for the unviable ones (I) and the total viability (VIATO) in the four categories. The above indicates that the essential internal tissues of the seeds were differentially affected by the combination of the tetrazolium concentrations with the evaluated staining times, at the level of the viable and doubtful seeds. These results are consistent with those reported in Glycine max seeds (Mercado and Delgado, 2018) and in Coffea arabica (Fantazzini et al., 2020), when evaluating different concentrations and staining times.
Table 3. Mean viability and germination values of the seeds (%) according to the tetrazolium test, at different concentrations and staining times in B. hispida (Thunb.) Cogn., in Montería - Colombia.
| Treatments | Viables (V) | Inviables (I) | Doubtful (D) | VIATO (V+0.5D) |
|---|---|---|---|---|
| 0.5% tetrazolium+2 h | 90.0 ab | 1.0 a | 9.0 ab | 94.5 a |
| 0.5% tetrazolium+3 h | 93.0 a | 2.0 a | 5.0 b | 95.5 a |
| 1.0% tetrazolium+2 h | 80.0 b | 1.0 a | 19.0 a | 89.5 a |
| 1.0% tetrazolium+3 h | 94.0 a | 1.0 a | 5.0 b | 96.5 a |
| 1.5% tetrazolium+2 h | 91.5 ab | 2.0 a | 6.5 b | 94.7 a |
| 1.5% tetrazolium+3 h | 88.0 ab | 2.5 a | 9.5 ab | 92.7 a |
| Germination test | 100.0 a | 100.0 a | ||
| Anova (P>F) | 0.027* | 0.10NS | 0.007** | 0.11NS |
| CV (%) | 6.23 | 18.1 | 34.31 | 3.68 |
| Normality (P>F) | 0.27ns | 0.13ns | 0.06ns | 0.25ns |
| Hom. Variance (P>F) | 0.25ns | 0.22ns | 0.08ns | 0.30ns |
Means with different letters indicate significant differences according to Tukey test (P≤0.05); NS not significant; * significant at P≤0.05; ** significant at P≤0.01; No VIATO (V + 0.5D): is the total of viable seeds (V) plus half of the doubtful seeds (D); CV: Coefficient of variation.
The highest percentages of viable seeds (Tab. 3) were detected in treatments 1.0% tetrazolium+3 h and 0.5% tetrazolium+3 h, with averages of 94 and 93%, respectively; these treatments presented difference with 1.0% tetrazolium+2 h (P<0.05). Similar trend for the means of viable seeds was found in doubtful seeds (D), which was to be expected since the treatments for non-viable seeds (I) did not present a significant statistical effect and their percentages were less than 2.6%. The presence of inviable seeds is due to degradation of cell membranes by lipid peroxidation and non-enzymatic peroxidation, which are factors that contribute to the degradation of seed viability (Hidayat and Ridhawati, 2020). The results of the comparison of means for the category of total viability of seeds (VIATO = V + 0.5D), in addition to confirming the results of the F test of the analysis of variance, absence of statistical differences between the means, allow us to infer that the combination of 0.5% tetrazolium with 2 h of staining is sufficient to determine the viability and vigor of the seeds, due to the intense, total and uniform staining in all the internal parts of the seed with the highest economy in tetrazolium and staining time (Fig. 2).Similar results have been reported in other studies (Mercado and Delgado, 2018; Fantazzini et al., 2020; Hidayat and Ridhawati, 2020).
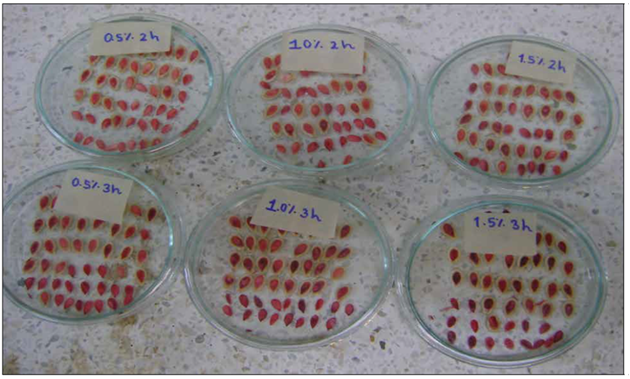
Figure 2. Intensity of staining at different concentrations and staining times in the tetrazolium test in B. hispida seeds. (Thunb.) Cogn.
Based on the above, 0.5% tetrazolium+2 h is the best combination of tetrazolium concentration and exposure time by allowing proper staining of living seed tissues, without impairing the visualization of viability, due to the concentration of 0.5% at 2 and 3 h, they cause a similar physiological effect in determining the viability of the seeds (Fantazzini et al., 2020; Hidayat and Ridhawati, 2020). In addition, the first two hours of water absorption by the seed are important, as they relate to enzymatic activity and, therefore, to the final coloration (Lima et al., 2010).
The coefficients of variation obtained in the study for the viable seed (V) and total viability (VIATO) categories indicate that the experimental technique was excellent and therefore the results of the study are reliable, as the values of the coefficients of variation resulted from 3.68% and 6.23%, for VIATO and V, respectively (Fantazzini et al., 2020). Other studies have reported CV values of 8 to 10% (Pinto et al., 2009; Souza et al., 2010; Lima et al., 2010).
Seed germination test
Table 4 contains the mean values for the physiological parameters of germination of B. hispida seeds. It can be seen that under mesh house conditions, the seeds started DIG at 6±0.02 days after sowing (dds), with a process duration time (DDG) of 13±1.1 and the end of the process germination from sowing (DFG) of 19±1.5. These times gave rise to a PG of 100±0.0%, IVG of 0.22±0.001 seedlings/day, GDM of 5.26±0.01% seedlings per day, VP of 5.33±0.12% of seedlings in one day and VG of 28±0.15. These germination parameters allow us to suggest that the B. hispida seed used in the study is of high physiological quality.
Table 4. Physiological parameters of the germination of B. hispida seeds. [(Thunb.) Cogn] in mesh house, Monteria - Colombia.
| DIG (#) | DFG (#) | DDG (#) | PG (%) | IVG (seedlings/d) | GDM (%/d) | VP (max.%/day) | VG (adim.) |
|---|---|---|---|---|---|---|---|
| 6±0.02 | 19±1.5 | 13±1.1 | 100±0.0 | 0.22±0.001 | 5.26±0.01 | 5.33±0.12 | 28±0.15 |
DIG: number of days at the beginning of germination; DFG: number of days at the end of germination; DDG: number of days during germination; PG: percentage of accumulated germination; IVG: germination speed index (plants / day); GDM: average daily germination percentage; VP: maximum germination percentage in one day; VG: germination value, obtained as the product of GDM×VP (dimensionless: adim.).
The PG obtained in house mesh was 100% (Tab. 3) and compared to the set of tetrazolium treatments there were no differences (P≥0.05) in relation to the VIATO variable. This shows the reliability of the tetrazolium test in the estimation of seed viability and high correlation (Fantazzini et al., 2020) and makes it possible to affirm that with a concentration of 0.5% tetrazolium chloride, a staining time of 2 h is sufficient to know the physiological quality of the seed of this species.
CONCLUSION
The morphometrics characteristics of thickness, length and width of the seeds showed scarce phenotypic variation, in comparison with the weight, density and volume of a seed.
The seed is ovoid and flattened, in its internal structure are the testa, seminal cover, micropyle, cotyledons and the embryonic axis wrapped in a thin protective membrane, which makes it waterproof.
The concentration of 0.5% tetrazolium, with a 2-hour staining dip, is sufficient to determine the viability of the B. hispida seed.