INTRODUCTION
Apples have high economic and nutritional value and are grown in many countries. The Department of Boyaca is important in the exploitation of deciduous crops in Colombia, including apples, japanese plums, peaches and pears, mainly because of the edaphoclimatic conditions of this department (Puentes et al., 2008; Casierra-Posada et al., 2017; Orduz-Rios et al., 2020). However, domestic apple production is well below national demand, resulting in large import volumes of apples from Chile. According to Puentes et al. (2008), the fundamental cause of problems in deciduous fruit trees in Colombia lies in the lack of cultivation planning. Mainly because the development of these fruit trees in the tropics is affected by the absence of chilling temperatures and long days (Fischer, 2013). However, there have been important advances in the use of new varieties with low chilling requirements and easy acclimatization to high-altitude tropics. Therefore, the study of deciduous fruit trees under tropical conditions is important for understanding the behavior of established varieties production factors that are necessary for each location to achieve good crop management (Puentes et al., 2008).
The most important apple variety in Colombia is Anna. It requires 300-400 chilling hours. The epidermis has red stripes where it receives sunlight and a yellow color on the other side. The pulp is creamy white with a medium texture and a sweet acid flavor. The fruit is medium-sized and has an elongated shape. In Colombia, two harvests are possible in areas with a bimodal precipitation regimen (Campos, 2013). However, there are few studies for this variety.
The growth and development of fruits are processes with biological and commercial importance (Malladi, 2020) that, must be modeled to design management strategiesfor apple orchards (Hester and Cacho, 2003). However, there are no recent scientific reports on the growth and development of apples in Colombia. This information would provide objective management recommendations for pruning, fruit thinning, fertilization, irrigation, harvesting, and other factors. One of the few studies available was conducted by Casierra-Posada et al. (2003), who reported results of a basic analysis of the growth of apples cv. Anna under the climatic conditions of Paipa, Boyaca by determining the weight and diameter of fruits and concluding that this fruit has a simple sigmoid growth curve. Likewise, Atay et al. (2010) reported that the growth of apples in temperate zones also follows a simple sigmoid pattern. However, Magein (1989) found that the growth of apples follows a double sigmoid curve, with a slow growth phase, attributed more to changes in the physiology of trees than to the physiology of fruits. In the world, new apple cultivars have been released that are adapted to a range of weather conditions. Some research has been conducted with respect to adaptation to local weather conditions and a few non-mathematical models have been developed (Chaves et al., 2017).
The apple is largely derived from non-ovarian tissues. Therefore, the regulation of fruit growth in apples is probably different from that of other models of fleshy fruit species. Fruit growth is an integration of multiple processes that are regulated through developmental factors, phytohormones, and the availability of metabolic resources (Malladi, 2020). These factors influence growth in different ways during the various stages of development and in the different tissues (Malladi, 2020). Nowadays, fruit quality is one of the main concerns of consumers (Fathizadeh et al., 2021). Quality depends to a large extent on the optimal harvest time of apples, which is derived from knowledge on the behavior of growth parameters. During ripening, there are changes in color, reductions in firmness and starch content, increases in soluble sugars, and decreases in acidity (Costa et al., 2010; Jing and Malladi, 2020). For example, the sugar content in apples is related to the development of the fruit (Zhang et al., 2015). Organic acids decrease as the fruit matures, and in combination with sugars and volatile compounds, determine flavor (Liu et al., 2016).
Therefore, the objective of this research was to analyze the growth and the main physicochemical changes in apple (Malus domestica Borkh) cv. Anna under conditions of a tropical cold climate at high altitudes.
MATERIALS AND METHODS
This study was carried out in a commercial orchard of deciduous fruit trees on the “Tunguavita” farm of the Universidad Pedagógica y Tecnológica de Colombia (UPTC), municipality of Paipa (Boyaca - Colombia), between March and June of 2016. Tis farm is located at 5°45' N and 73°45' W, at 2,525 m a.s.l. Climatic conditions during the experiment are presented in table 1. The laboratory phase was carried out in the plant physiology laboratory of the Faculty of Agricultural Sciences of the same university.
Table 1 Temperature and precipitation during the experiment.
| Month | Minimum (ºC) | Maximum (ºC) | Mean (ºC) | Precipitation (mm) |
|---|---|---|---|---|
| March | 7.7 | 26.0 | 16.9 | 34.6 |
| April | 10.4 | 23.0 | 16.7 | 197.5 |
| May | 8.8 | 22.1 | 15.5 | 112.7 |
| June | 6.9 | 21.2 | 14.1 | 25.7 |
Twenty apple trees (Malus domesticaBorkh) cv. Anna (Golden Delicious×Adassim Red) on MM106 rootstock, approximately 5-7 years old, planted at distances of 2 m between plants and 3 m between rows, and with production pruning were selected. The trees were trained to a central leader system, without fruit thinning. They were fertilized with mineral nutrients and organic matter according to the recommendations for the zone and based on soil analysis, and apple trees were supplemented with foliar fertilization. Drip irrigation water at regular intervals in the vegetation period with four drippers/tree of 8 L h-1 was used. Approximately 200 fruits were collected from initial flowers from each of the trees, which were previously marked in the anthesis stage. Harvesting of the fruits was done randomly from day 15 after anthesis (DAA) and every 15 days until harvest. Equation 1 was used to calculate growing degree days (GDD):
where Tmax and Tmin are, respectively, maximum and minimum daily air temperatures, and Tbase is the temperature at which the metabolic process in apples is at its lowest point, which was assumed as 7.22°C according to Chaves et al. (2017).
The following variables were determined in each of the samplings. The fresh weight was determined with a precision balance with an approximation of 0.01 g. Dry weight was determined by leaving the fruits in a muffle furnace at 75º C until a constant weight was obtained. The absolute growth rate (AGR) and relative growth rate (RGR) of dry weight were calculated following the methodology of Ochoa-Vargas et al. (2016). The polar diameter and equatorial diameter were determined with a 0.01 mm digital caliper.
To determine the respiration rate (mg CO2 kg-1 h-1), approximately 200 g of fruits were sealed in a 2 L hermetic chambers containing an infrared CO2 sensor. This was connected to an interface system (Labquest 1), and data was recorded every 5 s for 5 min (Africano-Pérez et al., 2016).
The fruit firmness (N) was obtained by using a PCE-PTR200 digital penetrometer with an approximation of 0.05 N. The color index of epidermis (background color) and pulp was calculated with equation 2 using the measurement of the L*, a* and b* parameters with a CR 410 digital colorimeter (Konica Minolta brand, Hong Kong):
The total soluble solids (TSS) were measured with a Hanna brand digital refractometer at a precision of 0.1°Brix. The total titratable acidity (TTA, % malic acid) was obtained with acid-base titration according to Mariño-González et al. (2019).
The data were presented as the mean ± standard error from 10 repetitions for the physical variables, each repetition composed of a fruit, while four repetitions were used for the chemical determinations. The behavior of each variable with respect to thermal time was plotted and the statistical models with the best fit were determined. The growth curves and rates were obtained with functional analysis, following the methodology used by Ochoa-Vargas et al. (2016) with SAS v. 9.2e (Cary, N.C).
RESULTS AND DISCUSSION
Growth analysis
Dry weight and growth rates: The apple fruits had a simple sigmoid type growth explained by a logistic growth model (Fig. 1; Tab. 2). Three growth phases were identified; in the first phase a slow growth was observed up to 159.61 GDD (30 DAA). At this point, the fruits gained in dry weight 0.732±0.026 g, and there was a stage of rapid growth that ended at 823.59 GDD (90 DAA); at which point, the fruits increased in dry matter 8.56±0.443 g. Finally, the ripening phase ended with the harvest at 892.37 GDD (100 DAA), where the growth was less with a gain in dry weight of 9.72±0.358 g. Casierra-Posada et al. (2003) found that developmentofthe ‘Anna’applefruitlasted120days in the region. This longer duration could mainly be due to differences in climate; these authors reported 13.8ºC, but in our study, the mean temperature was 2ºC higher (Tab. 1).
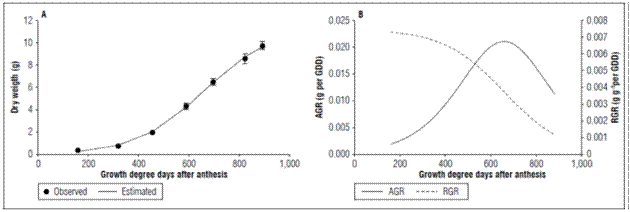
Figure 1. Behavior of A. Dry weight, B. Absolute growth rate (AGR) and Relative growth rate (RGR) of apples cv. Anna under high altitude tropical conditions. The vertical bars in figure 1A indicate the standard error (n=10).
Table 2. Fitting equations to the logistic model for the growth variables of the apple cv. Anna under high-tropical conditions.
| Parameter | Equations | R 2 |
|---|---|---|
| Dry weight of the fruit | Y= 11.274/1+e-0.007471*(GDD-657.89) | 0.9988** |
| Fresh fruit weight | Y= 78.669/1+e-0.0073213*(GDD-651.6) | 0.9979** |
| Polar diameter | Y= 55.758/1+e-0.0317977*(GDD-535.426) | 0.9912** |
| Equatorial diameter | Y= 48.6469/1+e-0.02576*(GDD-350.259) | 0.9913** |
** Significant models (P≤0.01).
The growth rates confirmed the simple sigmoid behavior since, in the first phase, the AGR had a slow rise with a value for 319.79 GDD of 0.0069 g per GDD, and the RGR had a slow decrease at this same point of 0.0058 g g-1 per GDD; then, in the rapid growth phase, the AGR increased rapidly, reached its maximum value at 654.91 GDD with a value of 0.0211 g per GDD and then decreased. The RGR had a rapid decrease for this same point, which confirmed the rapid gain in dry weight in the second phase. In the maturation stage, the AGR showed a rapid decrease with a value at 892.37 GDD of 0.011 g perGDDand the RGR showed a value at that same point of 0.0011 g g-1 per GDD, indicating the lowest rate accumulation of dry weight during the ripening phase (Fig. 1B). This means that the maximum growth rate occurred in the second stage of development. This agrees with that observed in lulo fruits (Almanza-Merchán et al., 2016), pears (Molina-Ochoa et al., 2016) and Ultra Red Gala apples (Yuri et al., 2011). In this latter study, the AGR and RGR had similar trends to those found in cv. Anna, with the difference that, in the first growth phase, these rates indicated a higher growth speed in Ultra Red Gala apples.
The fresh fruit weight was also described by a logistic model with the simple sigmoid pattern (Fig. 2A; Tab. 2). Three phases of fruit growth were identified. In the first phase up to 319.79 GDD, a slow growth was observed; at this point, the fruits accumulated 5.865±0.169 g of fresh weight. In the second phase an increase in growth speed was observed up to 823.59 GDD (90 DAA) with a fresh weight gain of 63.664±3.394 g. For the third growth phase, which culminated with the fruit harvest, the growth slowly decreased to 892.37 GDD with a fresh weight gain of 66.088±2.544 g.
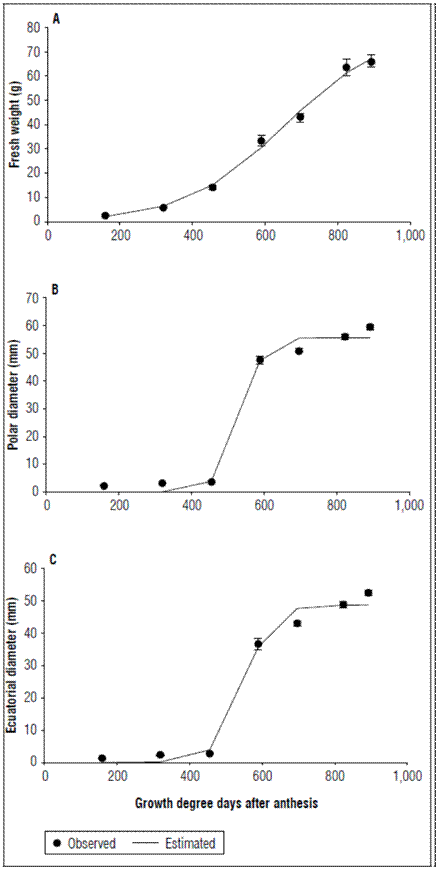
Figure 2. Behavior of A. Fresh weight, B. Polar diameter and C. Equatorial diameter of apples cv. Anna under conditions high-altitude tropics. The vertical bars at each mean indicate the standard error (n= 10).
In Ultra Red Gala apples, growth was also simple sigmoid but was adjusted to a Gompertz model (Yuri et al., 2011), while ‘Anna’ was adjusted to a polynomial regression (Casierra-Posada et al., 2003). The simple sigmoid type growth found in cv. Anna for dry and fresh weight is a manifestation of the three growth periods of apples: a first period of intense cell production; a second period of cell elongation, the vacuoles increase in size and starch, organic acids, sugars and other components begin to accumulate; and the last phase has the highest growth speed (Malladi, 2020), with a final maturation phase, where the maximum accumulation of weight is reached.
Photosynthesis plays a fundamental role in dry weight gain because of the translocation of photoassimilates from other plant parts to fruits. This remobilization of photoassimilates corresponds to between 40 and 50% of the weight of fruits as reported for mango (Castro Neto and Reinhardt, 2003). However, it is a process that largely depends on the sink activity of fruits, which is influenced by the hormonal synthesis of seeds (Balaguera-López et al., 2020). In apples, miR172 has been associated with the regulation of growth and final fruit size (Yao et al., 2015). Lower miR172 levels were associated with increased fruit growth; for this reason, overexpression of miR172p (one of several active miR172s) in transgenic 'Royal Gala' plants results in a representative reduction in fruit size (Yao et al., 2015). The same authors suggested that the basal regions of floral organs, particularly sepals, contribute greatly to the development of major fleshy tissues of the apples.
Monitoring fruit growth and apple size is important for several reasons: prediction of potential fruit size and yield, as well as for use in crop simulation models (Zadravec et al., 2013). Early prediction models combine fruit counts and size measurements with pre-calculated growth curves and correlations between fruit diameter and weight (Wulfsohn et al., 2012). In addition, the models establish agronomic practices such as fertilization, irrigation, thinning, pruning, among other reasons.
The polar diameter was fitted to a logistic model. During the first 455.39 GDD (45 DAA), the increase was not very representative, and the fruits reached 3.659±0.0593 mm. From 455.39 GDD, there was a marked increase in diameter up to 589.32 GDD (60 DAA), at which point the diameter was 47.622±1.4351 mm. An increase in this parameter was still monitored until harvest, when the fruits reached 59.5±0.9409 mm (Fig. 2B; Tab. 2). The equatorial diameter at the time of harvest was 52.393±1.4351 mm and was similar to the polar diameter (Fig. 2C; Tab. 2), but was always smaller, an indication of the characteristic shape of the fruit of this cultivar, which tends to be an elongated fruit because of the tropical high altitudinal conditions. The values of the diameters were notably lower than those reported for varieties such as Golden Delicious Reinders and Fuji Kiku 8 (Zadravec et al., 2013). These same authors indicated that these discrepancies in fruit diameter and weight can be explained by genetic factors (cultivars, strains) location, and dependence on environmental conditions.
The increase in the two diameters from 455.39 to 589.32 GDD was abrupt, while, in the fresh and dry weights, this increase was not so marked. This was perhaps due to the fact that the fruits increased the void spaces in the pulp in the expansion phase. This is a phenomenon that occurs in apples and can determine fruit firmness (Saei et al., 2011).The development of void spaces is one of the three main processes that determines growth, along with cell production and expansion. However, the development of void spaces remains a little known process despite its relatively significant contribution to the final size of fruits (Malladi, 2020).
Physicochemical behavior of fruits
The respiratory rates showed a continuous decrease during the development of the fruits, whose behavior was adjusted to a second degree polynomial. At 159.61 GDD, there was a respiratory rate of 61.93±6.79 mg CO2 kg-1 h-1 corresponding to the fruit formation phase. At 892.37 GDD, the lowest rate corresponded to the physiological maturity phase, 4.23±0.16 mg CO2 kg-1 h-1 (Fig. 3A).
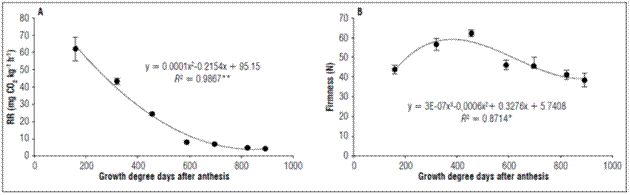
Figure 3. Behavior of A. Respiratory rate and B. Firmness of apples cv. Anna under conditions in high-altitude tropics. The vertical bars in each mean indicate the standard error (n=4).
In the first phase of fruit development, at high respiratory rate supplies the energy requirements for the cell division process, a process that determines the relative rate of cell production and reaches a maximum of around 0.26 cells per cell per day during the initial period of fruit growth in apple (Dash et al., 2012). Hexokinase activity and the abundance of hexokinase gene transcripts is higher during early fruit growth (Li et al., 2012). This flow towards glycolysis and subsequent respiration is essential to meeting the demands for energy and carbon skeletons in this phase of development (Malladi, 2020). The glycolytic flow is high during the early development of fruits (Beshir et al., 2017) and decreases in later stages (Li et al., 2016), which explains the respiratory behavior obtained in the cv. Anna.
Respiratory intensity is a good physiological index to determine fruit harvest; when the respiration rate reaches its minimum level, fruits are in full development. This behavior could be observed in apples; as fruit growth increases, a decrease in respiration is seen. The apple fruit reached physiological maturity at 892.37 GDD. At this point, the lowest respiratory rate occurred when the fruits were physiologically ready to be harvested.
The behavior of the firmness was characterized by an increase in the first 455.39 GDD, when a value of 62.19±1.76 N was obtained, then a very marked decrease up to 823.59 GDD with a value of 41.16±2.43 N, with a gradual decrease up to 892.37 GDD with a value of 38.38±3.48 N, which adjusted to a third-degree polynomial (Fig. 3B). Firmness is a primary measure of apple fruit texture and is the key determinant of eating quality for apples. There is very limited information of firmness in pre-harvest and at harvest. These are causes of variations in fruit quality in the marketplace (Saei et al., 2011). These same authors reported that firmness in the apples depends on variation in the strength of the tissue caused by differences in the size of the cells, the number of cells, the intercellular air space, and the material of the cell wall per unit volume of the fruit.
The increase in firmness that is observed in apples in the first growth phase, also observed in araza, may be associated with the synthesis of protopectin (Hernández et al., 2007). For the second growth phase of the apples, a decrease in firmness was seen. The decrease in the firmness of the fruits coincides with the dissolution or hydrolysis of the middle lamina and cell wall, resulting in a reduction in intercellular adhesion, depolymerization, and solubilization of hemicellulosic and pectic polysaccharides of the cell wall (Morais et al., 2008). Alterations in cell turgor and the degradation of starch reserves also lead to a loss of firmness (Kays, 2004). Similarly, in apples cv. Fuji, the highest firmness values occur between 30 and 58 days after flowering, with a continuous decrease until harvest (Sha et al., 2020).
The background color index of the epidermis increased as a function of time, going from a value of -7.99±0.1 at 159.61 GDD to -5.12±0.29 at 892.37 GDD. The color index of the pulp also increased throughout the development of the fruit, from a value of -5.61±0.293 at 159.61 GDD to -3.311±0.317 at 892.37 GDD (Fig. 4).
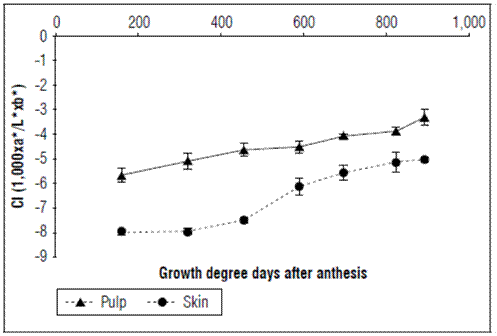
Figure 4. Behavior of the color index of the epidermis and pulp in apples cv. Anna under conditions in high-altitude tropics. The vertical bars in each mean indicate the standard error (n=10).
The epidermis of the fruit is red where it receives sunlight and yellow on the opposite side (Campos, 2013). The background color was measured since the red color of the coating is not a good indicator of ripening. The results indicated that, as fruits progress in development, the coloration of both the epidermis and the pulp increased linearly with respect to time. However, the values of the epidermis were below zero at harvest, indicating that they were still green. At postharvest, they can acquire yellow atonalities, although they are sometimes very weak, indicating that this cultivar has coloration problems, mainly because of environmental factors. This is a commercial limitation because yellow coloration is not attractive to consumers. In this regard, the color of the fruits is an important contribution for health and economic benefits (Zhu et al., 2019), influencing sales and acceptance by consumers (Iglesias et al., 2012). Finding solutions to improve the color of the Anna cultivar should be a priority to make these fruits more competitive that imported apples.
In apples, a color change is due to the degradation of chlorophylls and the synthesis of anthocyanins. The latter are dependent on light for their production (Iglesias et al., 2012), and can be affected by factors such as temperature, state maturity, and cultivars (Lin-Wang et al., 2011; Iglesias et al., 2012). This indicates that, in addition to genetics, ecophysiological factors are of great importance to attractive colorations in cv. Anna fruits. Cool nights are recommended prior to harvest since high temperatures can inhibit anthocyanin synthesis (Lancaster, 1992). Achieving an optimal skin color in apples is usually a problem in most southern European countries (Iglesias et al. 2012).
The total soluble solids increased linearly as a function of time: at 159.61 GDD, they had a value of 5.5±0.35ºBrix at 589.32 GDD a value of 6.975±0.33ºBrix was observed, and, at 892.37 GDD, the values was 8.58±0.37ºBrix (Fig. 5A).
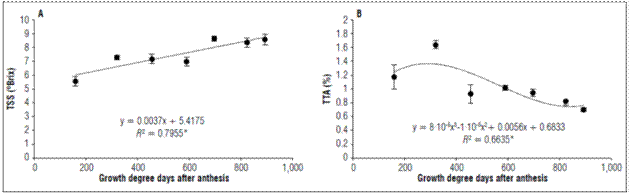
Figure 5. Behavior of A. Total soluble solids and B. Total titratable acidity of apples cv. Anna under conditions in high-altitude tropics. The vertical bars in each mean indicate the standard error (n=4).
A TSS increase in apples in the early stages is due to the arrival of sorbitol and sucrose via phloem (Sha et al., 2020). They are used for metabolism, and the surplus accumulates mainly as starch, towards the middle of fruit development (Malladi, 2020). Potentially, sucrose synthase activity can metabolize sucrose to generate the cellulose synthesis necessary for supplying new growth requirements cell wall, which is associated with rapid early growth in apples (Verbančičet al., 2018). In the maturation stage the increase in TSS is explained by the hydrolysis of various structural polysaccharides such as starch, pectins and other oligosaccharides of the cell wall, down to basic monomeric components, which solubilize in the aqueous phase and became part of the juice (Kays, 2004). The main sugars in apple fruits are fructose, sucrose, glucose and sorbitol (Filip et al., 2016). Sorbitol and sucrose stand out since their concentrations increase during fruit development. Towards the ripening stage sucrose is predominant (Sha et al., 2020). Sugars accumulation during ripening is associated with optimal edible quality.
The TTA during the development of the fruit was adjusted to a cubic regression, with increased up to 319.79 GDD where the acidity was 1.20±0.17%. Later there was a decrease until the harvest, when the fruits presented a TTA of 0.71±0.03% (Fig. 5B). Acids are also significantly responsible for flavor with a typical relationship between sugars and acids (Kays, 2004). For the apple ‘Anna’, this is one of the most important attractions for consumers.
Malic acid is the most abundant organic acid in apples and accumulates during development (Walker and Famiani, 2018). The malate concentration increases gradually during the early development of the fruit and reaches a peak towards the end of cell production during fruit growth (Zhang et al., 2010), as found in this study with a higher TTA at 319.79GDD(Fig. 5B).
It is assumed that acidity decreases as the ripening process advances, as is reported in three apple varieties, Gala, Golden Delicious and Princesa (Duran, 1983). The decrease in TTA is due to the use of organic acids during maturation as respiratory substrates and as carbon skeletons for the synthesis of new compounds (Kays, 2004).
CONCLUSIONS
The dry and fresh weights and the equatorial and polar diameters followed a simple sigmoid pattern. Thefirmnessdecreased from455.39GDDuntil892.37GDD(100DAA). The background color index of the epidermis and the pulp showed that the fruits began the growth phase with an intense green color that was lost as a function of development. The TSS had a linear increase with respect to the days after anthesis, and the TTA decreased from 319.79 GDD, coinciding with the stage of greater accumulation of dry weight. The respiratory rate of the fruits decreased continuously during the development of the fruits until the ripening phase.















