INTRODUCTION
The champa (Campomanesia lineatifolia R. & P., Mirtaceae) or “chamba” is a fruit tree whose fruits are edible berries. According to Silva and Fonseca (2016), this genus is characterized by its citrus-flavored fruits. It is an exotic fruit native to the Amazon rainforest that has considerable potential for commercialization and is currently being introduced in different markets in Colombia (Balaguera et al., 2009; Balaguera-López and Herrera, 2012a; Porras et al., 2020). This fruit has a high concentration of organic acids, sugars and volatile compounds (Balaguera and Herrera, 2012a), which makes it highly desirable for consumption, fresh or in processed products (Álvarez-Herrera et al., 2009a). In recent years, the champa has been reported as an important source of bioactive substances with great potential for use in the agricultural, pharmaceutical, cosmetic and food industries (Muños et al., 2015; Otálvaro-Álvarez et al., 2017).
However, the high perishability of champa fruit represents a major limitation of the product, which is responsible for considerable postharvest losses (Álvarez-Herrera et al., 2009a). In addition, its storage and marketing for fresh consumption have been limited by a lack of technological development (Muños et al., 2015). One way to reduce postharvest losses is to elucidate which processes are associated with ethylene during ripening for use as a basis for different technologies that reduce the biosynthesis and action of ethylene (Mariño-González et al., 2019) because ethylene is responsible for triggering processes at the physiological, biochemical and molecular levels (An et al., 2020), not only guaranteeing fruit ripening but also inducing senescence, an important cause of nutritional, physical and economic losses in agricultural products (Binder, 2008; Bapat et al., 2010). Therefore, several studies have looked at applications of ethylene and 1-methylcyclopropene (1-MCP) in fruits, which can done in a methodological way to determine the role of endogenous ethylene in ripening (Yang et al., 2013; Mariño-González et al., 2019).
1-MCP is a chemical compound that irreversibly occupies the membrane receptors of ethylene. At recommended doses, 1-MCP is not toxic to human beings and / or the environment; it is odorless and stable at room temperature. In addition, it is easy to apply and highly effective in protecting many agricultural species from the action of ethylene (Serek et al., 2006). It is not only considered as the main compound inhibiting ethylene action (In et al., 2013) but also as a commercial strategy commonly used for fruit and vegetable management (Lata et al., 2017). 1-MCP applications have generated delays in ripening processes in cape gooseberries (Physalis peruviana L.; Valdenegro et al., 2012), mangos (Razzaq et al., 2016), melons (Shi et al., 2014) and peaches (Mariño-González et al., 2019).
On the other hand, temperature is one of the most important factors that affect the quality of fresh produce. This is the reason why temperature management is a key tool for extending storage capacity and shelf-life in freshly harvested products, since it slows down both physiological and pathological deterioration with a decrease in metabolic processes, including respiration (Toivonen, 2016; Brasil and Siddiqui, 2018). Therefore, refrigeration is the most widely used and effective technology for reducing postharvest deterioration in fruit and vegetable products because it reduces commercial losses during storage (Lado et al., 2015). There is an optimal storage temperature for each product and the ideal temperature often depends on the geographical origin of the product (Brasil and Siddiqui, 2018). In champa fruits, there are no known studies involving the use of refrigeration. However, its use has been reported in different species: for instance, Obenland et al. (2013) found a better response in storage at 5ºC with respect to 20ºC in mandarins, cv. W. Murcott Afourer. In guava, an optimal storage temperature of 5-10ºC has been reported (Parra-Coronado, 2014). Nonetheless, refrigeration and 1-MCP are the main techniques for increasing the postharvest shelf-life of tomato fruits (Zou et al., 2018). In pear fruits, refrigeration was also used effectively in combination with 1-MCP (Cheng et al., 2019).
The objectives of this study included: First, evaluate the application of 1-MCP and ethylene, individually and in combination, as well as compounds involved in the regulation of champa fruits to understand ethylene-dependent ripening processes found in the fruit. Second, determine the effect of 1-MCP and low temperatures as strategies to prolong the postharvest conservation and shelf life of these fruits.
MATERIALS AND METHODS
Champa fruits (Campomanesia lineatifolia R. & P.), in stage 2 maturity (25% yellow-75% green; Balaguera-López and Herrera, 2012b), were collected in the municipality of Miraflores, Boyaca (Colombia), from approximately 5-7-year-old trees, without agronomic management, but free of phytosanitary issues. This municipality is located at 1,432 m a.s.l., 5º11'49''N and 73º08'47''W, with a mean temperature of 24ºC and 2,500 mm of annual rainfall. The experiments were carried out in the Plant Physiology laboratory of the Universidad Pedagógica y Tecnológica de Colombia, which is part of the Faculty of Agricultural Sciences of this university.
Two experiments were carried out: The first one evaluated the effect of 1-MCP and ethylene, whereas the second one evaluated the effect of 1-Methylcyclopropene (1-MCP), as well as refrigeration.
Experiment 1. 1-MCP and ethylene: A completely randomized design was used with four treatments, repeated four times for a total of 16 experimental units, each consisting of 500 g of fruit. Such treatments were: ethylene (1,000 µL L-1), 1-MCP (0.35 mg L-1), 1-MCP (0.35 mg L-1) + ethylene (1,000 µL L-1) and control without application.
2 L containers were used for the application of ethylene in which a solution of 1,000 µL L-1 of ethephon (Ethrel® 48 SL, Bayer CropScience) was prepared. The fruits were immersed in this solution for 10 min and then allowed to dry at room temperature. For the 1-MCP treatment, 2 L of a 0.35 mg L-1 solution of 1-MCP was made. The fruits were immersed for 10 min and then left to dry at room temperature. For the 1-MCP+ethylene treatment, ethylene was applied 2 d after treatment. The fruits were then arranged in polystyrene trays and stored at room temperature (16±2°C, 75±6% RH). This methodology was slightly modified from Mariño-González et al. (2019).
Experiment 2. 1-MCP and refrigeration: In a second experiment, four treatments in a 2×2 factorial arrangement were evaluated with a completely randomized design. The first factor pertained to the application of 1-MCP, while the second factor corresponded to storage temperature (2±0.4°C) and ambient (16±2°C). Each of the experimental units involved a polystyrene tray with 500 g of fruit. The 1-MCP was applied with the same procedure as in experiment 1.
In the two experiments, the following parameters were determined periodically during the storage process: until the fruits lost their organoleptic quality, which was visually estimated.
Determination of respiration rate: Approximately, 100 g of fruit were placed in a 2 L airtight chamber for 5 min. In the chamber, an infrared CO2 sensor was placed (VER CO2-BTA), which was connected to a Labquest data collection device (Vernier Software and Technology, Beaverton, OR). The respiration rate was then calculated in mg CO2 kg-1 h-1 taking into account the weight of the fruits as well as the volume of the chamber (Mariño-Gonzalez et al., 2019). The epidermis color index (CI): was calculated with the following equation (1):
The CIEL*a*b* system parameters L*, a* and b* were determined with a CR-410 colorimeter (Konica Minolta) with three inputs at the equatorial diameter. For weight loss (PP, %), a sample of approximately 100g of fruit was measured for fresh mass; and subsequently, the following equation was used (2):
where, P1 is initial fruit weight, and P2 final fruit weight.
Fruit firmness (N) was measured using a PCE-PTR200 penetrometer. Soluble solids (SS; ºBrix) were defined using a digital refractometer (Hanna brand, Woonsocket, RI). The titratable acidity (TA; % citric acid) was determined with acid-base titration with a 0.1 N NaOH solution.
The data obtained statistically analyzed using Shapiro-Wilk and Levene's test, followed by analysis of variance and Tukey's test for comparison of means with P≤0.05 (Mariño-Gonzalez et al., 2019). Analyses were then carried out using SPSS v. 19.
RESULTS AND DISCUSSION
Experiment 1. 1-MCP and ethylene
The respiration rate of the champa fruits decreased in all treatments in the first 3 d of storage. However, it then had a continuous, significant increase in all treatments, which is typical of its climacteric character (Álvarez et al., 2009b). The rate was significantly higher (P≤0.05) in the control fruits with the application of ethylene and, as a result, these fruits lost their organoleptic quality at 10 d after storage. The fruits with 1-MCP and 1-MCP+ethylene had a postharvest duration of 17 d and presented a maximum respiration rate of 60.1±4.2 and 58.07±6.4 mg CO2 kg-1 h-1, respectively. During storage, these two treatments had a similar behavior (Fig. 1A).
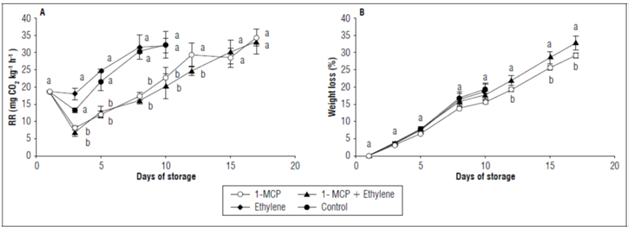
Figure 1. A) Respiration rate (RR) and B) weight loss of champa fruits under postharvest applications of 1-MCP and ethylene. The vertical bars in each mean value indicate the standard error (n=4). Means followed by different letters in each measurement indicate significant differences according to the Tukey's test (P<0.05).
The champa fruits with higher ethylene levels but without 1-MCP increased their respiration rate in a more characteristic way, because this hormone has the ability to stimulate respiration in fruits to induce the ripening process (Bapat et al., 2010; Kays, 2004), as found in peaches (Mariño-González et al., 2019). It has been found that ethephon can accelerate energy production, glycolytic metabolism and ethylene biosynthesis, processes that stimulate tissue respiration (Zhang et al., 2012). As a result of the inhibitory action on the receptors and the ethylene biosynthesis process (Balaguera-López et al., 2021), the application of 1-MCP was responsible for slowing down the respiratory rate in the champa fruits. This decrease in the respiratory rate with the ethylene inhibitor was responsible for a longer postharvest time of champa fruits, as reported by Daulagala and Daundasekera (2015) in avocado studies and by Cerqueira et al. (2009) in guava fruits. It is also important to note that the application of ethylene 2 d after 1-MCP did not reverse this effect. Respiration has a very important influence on fruit perishability, which indicates that 1-MCP may become a practical alternative for improving the postharvest life and quality of champa fruits.
Weight loss in all fruits increased progressively. While the fruits in all treatments were in commercial quality (10 d), there were no statistical differences. However, after this point, the fruits with 1-MCP+ethylene significantly lost weight. Eventually, losses for these two treatments were 32.7±0.8 and 29.1±2%, respectively (Fig. 1B). Since this fruit is characterized by considerable weight loss (Balaguera-López and Herrera, 2012b), this may explain why there were no differences between treatments until day 10. The fact that the fruits with 1-MCP, with and without ethylene, presented the greatest weight loss was due to the fact that they remained at room temperature for longer because they had a longer postharvest duration, as was reported by Balaguera-López and Herrera (2012b).
The color index of the epidermis had a continuous for the storage. This increase represented a change in color from green to yellow. The CI was statistically higher in the fruits with ethylene and in control fruits with values higher than 4. The fruits with 1-MCP presented a longer postharvest duration and, a slower color change but had similar values from 12 d to the end of storage (17 d). At this point, the CI was close to 3.7 (Fig. 2A).
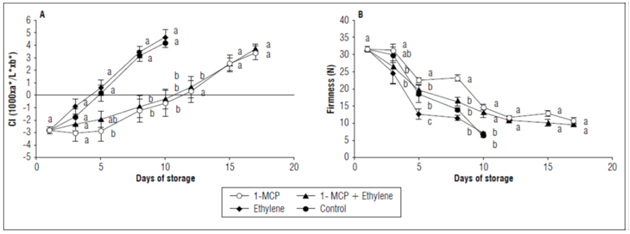
Figure 2. A) Epidermis color index and B) firmness of champa fruits under postharvest applications of 1-MCP and ethylene. The vertical bars in each mean value indicate the standard error (n=4). Means followed by different letters in each measurement indicate significant differences according to the Tukey's test (P<0.05).
The untreated champa fruits, as well as the fruits with ethylene presented a greater and rapid change in the epidermis color. This change can be explained not only by the high level that these fruits were assumed to have -which stimulates the degradation of chlorophylls through chlorophyllase enzymes- but also by to the increase in the synthesis of carotenoids, which favors the characteristic yellow color of this species (Álvarez-Herrera et al., 2009b), highlighting the importance of this hormone in the regulation of fruit color. For the application of 1-MCP, the significant delay in CI indicates the effectiveness of this compound at avoiding the action of ethylene, thus delaying the activation of processes related to color change in the champa fruits. This result has also been evidenced in feijoa fruits (Rupavatharam et al., 2015).
Changes in color can be ethylene-dependent or ethylene-independent (Lelievre et al., 1997). Therefore, the results presented here indicate that, in the champa fruits, color was ethylene-dependent, as found in peaches and cape gooseberries (Mariño-González et al., 2019).
A loss of firmness was observed during storage in all fruits. Significant differences (P≤0.05) were observed from day 3 to 10 d of storage. It was evident that the fruits with 1-MCP, alone or with ethylene, delayed softening, and eventually, the fruits presented more than 15 N (Fig. 2B). 1-MCP affects biosynthesis, signaling, and, as a result, processes that depend on ethylene during ripening, in this specific case, firmness. This is done by decreasing the activity of hydrolase enzymes that mainly affect the cell wall, including polygalacturonase, whose activity is reported for this species (Balaguera-López and Herrera, 2012a). Consistently, several studies have indicated that 1-MCP decreases softening in other Myrtaceae fruits, such as guava and feijoa (Cerqueira et al., 2009; Rupavatharam et al., 2015). Enzymes that are involved in the loss firmness such as polygalacturonase, pectin methylesterase, endo-β-1,4-glucanase, α-arabinosidase and β-galactosidase, have higher activity with ethylene applications because the expression of related genes increases (Nishiyama et al., 2007; Pech et al., 2008). This may explain the greater softening in the control and ethylene-added champa fruits at the beginning of storage, was an indication that firmness in this species is apparently regulated by ethylene.
An increase in fruit TA was observed up to 5 d. of storage and then decreased until loss of commercial quality. There were statistical differences only at 10 d since the control fruits and fruits with ethylene had the lowest TA. The fruits with 1-MCP -alone or in combination- continued to lose TA. However, at 17 d of storage, they presented the lowest values throughout all storage process, with a TA of approximately 2% (Fig. 3A).
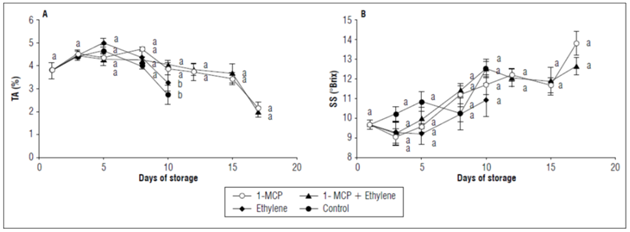
Figure 3. A)Titratable acidity and B) Soluble solids of champa fruits under postharvest applications of 1-MCP and ethylene. Vertical bars in each mean value indicate the standard error (n=4). Means followed by different letters in each measurement indicate significant differences according to the Tukey's test (P<0.05).
This fruit is characterized by high acidity values (about 3%), which can increase during ripening (Balaguera-López and Herrera, 2012a, b). The decrease observed after 5 d in all treatments may have been the result of increased metabolic expenditure, which includes respiration and gluconeogenesis (Kays, 2004; Toivonen, 2016). However, it is important to indicate that at 10 d, ethylene stimulates the expenditure of organic acids, whereas the use of 1-MCP delays it. This result was closely related to the one obtained in respiration (Fig. 1A), which also showed that TA metabolism may be regulated by ethylene. Barreto et al. (2017) showed similar results in peaches, finding higher acidity in fruits treated with 1-MCP than in fruits treated with ethylene, the latter accelerating the loss of acidity in these fruits. It was found that 1-MCP decreased the degradation of organic acids in guava fruits (Singh and Pal, 2008).
There were no significant differences in any of the samplings and the tendency was to increase the SS. At the beginning of the experiment, the fruits presented less than 10ºBrix, whereas the fruits with 1-MCP accumulated approximately 14 ºBrix (Fig. 3B). This increase in SS may have been due to the hydrolysis of starch and reserve polysaccharides (Kays, 2004). This metabolism is expected to be regulated by ethylene. However, since acids are constituents of SS and, in this study, accounted for between 28 and 54% of the SS value, they can easily affect them. Therefore, there was not enough evidence to affirm or rule out that SS were regulated by ethylene in the present study.
Effect of 1-MCP and refrigeration
Significant differences (P≤0.05) were observed from day 3 to 19 d in the respiration rate. Thus, there was a decrease between day 1 and 3 d. The refrigerated fruits (with and without 1-MCP) maintained a constant respiration rate throughout storage, whereas the fruits at room temperature showed a continuous increase that was related to the postharvest duration. The control fruits lost their organoleptic quality at 12 d of storage, and their respiration rate was 59.1±4.2 mg CO2 kg-1 h-1. In contrast, the fruits at room temperature with 1-MCP lasted until 19 d, while the refrigerated fruits remained for 26 d, and their respiration rate did not exceed 13 mg CO2 kg-1 h-1(Fig. 4A).
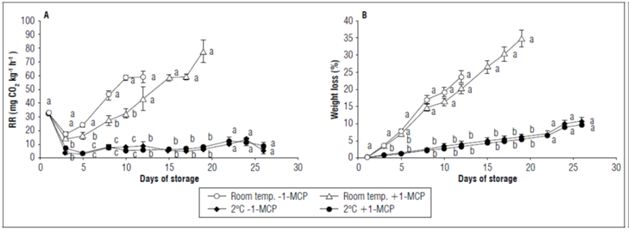
Figure 4. Effect of postharvest application of 1-MCP and refrigeration on: A) respiration rate and B) weight loss of champa fruits. Vertical bars in each mean indicate the standard error (n=4). Means followed by different letters in each measurement indicate significant differences according to the Tukey's test (P<0.05).
The champa fruits with 1-MCP at room temperature decreased their respiration rate, as compared to the control fruits, through inhibition of the ethylene receptors (Lata et al., 2017), as previously mentioned. However, there was no effect when 2°C refrigeration was used, which was possibly due to the fact that refrigeration may decrease the affinity or interaction of 1-MCP with ethylene receptors, or perhaps the decrease in the solubility of 1-MCP in the cell, as mentioned by Mir et al. (2001) and Villalobos et al. (2011). It has been mentioned that refrigeration is used in order to decrease the respiration rate, thus preserving the quality of the fruit and delaying senescence. With this decrease in respiration, both the metabolic and enzymatic activities of fruits are reduced (Pereira et al., 2013). Wu et al. (2015) reported that at postharvest, the activities of antioxidant enzymes (CAT, SOD and POD) are very important for scavenging ROS to protect cell membranes and delay senescence. This explains the duration of 26 d in the use of refrigeration, as compared to the 12 d in the fruits without treatments. Concordant results were found by Balaguera-López et al. (2015) in cape gooseberry fruits, and by Carrillo et al. (2011) in Eugenia stipitate (araza) fruits, where refrigeration reduced the respiration rate and extended the postharvest conservation.
Continued weight loss was evident during storage. The fruits under refrigeration -regardless of the addition of 1-MCP- had statistically the lowest weight loss, resulting in a 11% loss of their weight after 26 d. The control fruits lost 23.7±1.7% of their weight, whereas the fruits at room temperature with 1-MCP lost 34.8±2.5% (Fig. 4B). Refrigeration, in addition to decreasing respiration in the champa fruits, may have delayed deterioration and thus water loss by transpiration, as previously mentioned in other studies (Toivonen, 2016; Brasil and Siddiqui, 2018). Likewise, Carrillo et al. (2011) found that 1-MCP, together with storage at 7°C, reduced weight loss in araza fruits.
The color index of the control fruits changed rapidly, and a value of 4.1±0.7 was obtained at 12 d. The fruits at room temperature with the addition of 1-MCP had statistically similar values to the fruits under refrigeration until 12 d. However, they had a marked increase in their CI until 19 d. The fruits under refrigerated storage, with and without 1-MCP, slowly changed the epidermis color with a CI greater than zero toward the end of the storage period (Fig. 5A).
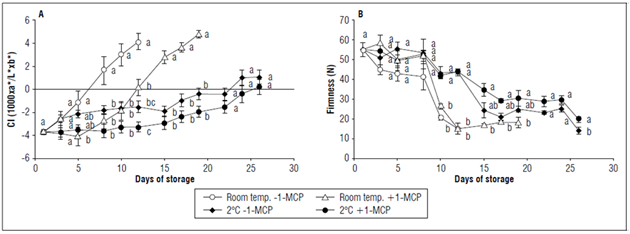
Figure 5. Effect of postharvest application of 1-MCP and refrigeration on: A) the color index of the epidermis and B) the firmness of champa fruits. The vertical bars in each mean indicate the standard error (n=4). Means followed by different letters in each measurement indicate significant differences according to the Tukey's test (P<0.05).
The 1-MCP was efficient when was compared with the control. This result may have been due to the fact that this compound decreases the activity of the enzyme chlorophyllase and therefore the loss of chlorophyll (Lata et al., 2017), delaying color change. However, when 1-MCP was used at 2°C, there was no significant effect in relation to refrigeration alone, perhaps because of the significant decrease in metabolism at this temperature.
It is possible that the lower CI in the refrigerated fruits was due to a lower loss of chlorophylls and less accumulation of carotenoids, as a result of a slower ripening process. In addition, it is well known that low storage temperatures reduce metabolic activity (Toivonen, 2016). In tomato fruits, it was found that refrigerated storage delayed color change because of a lower accumulation of carotenoids (Rugkong et al., 2011). It is important to clarify that there were no visible symptoms of cold damage during storage such as peel pitting or external browning. However, evaluations with shelf-life periods are suggested to be completely sure that 2ºC does not cause physiopathologies in champa fruits.
The treatments at room temperature showed a more accelerated loss of firmness than the treatments under refrigeration. The treatment with 1-MCP together with refrigeration maintained greater firmness of the fruits during the storage period and presented a final firmness of 19.9±1.5N (Fig. 5B).
It is known that one of the main causes of softening in fruits is the activity of enzymes that hydrolyze polysaccharides (Kays, 2004). It has been found that the depolymerization of pectins and xyloglucan are regulated positively by ethylene (Nishiyama et al., 2007; Pech et al., 2008). For this reason, the champa fruits with 1-MCP had slightly higher firmness and a longer postharvest duration than the control fruits. However, the greatest favorable effect on firmness was generated by a low temperature, possibly because of the fact that low temperatures have the capacity to decrease the genes expression involved in process of ethylene biosynthesis, which leads to decreased ethylene biosynthesis and reduced cell wall degradation, as has been reported in tomato fruits (Rugkong et al., 2011). In the same species, refrigeration decreased the activity of polygalacturonase and pectin methyl esterase 1 enzymes (Rugkong et al., 2010). This shows the importance of refrigeration in maintaining champa fruit firmness.
For titratable acidity, there were significant statistical differences from 8 to 19 d of storage. Although there was an increase in acidity in the first days of storage, a decrease was later evident; however, it was more marked in the fruits at room temperature. At the end of the storage period, the refrigerated fruit had higher TA values, around 3.5% (Fig. 6A). These results indicate the favorable effect of low temperatures in the decrease of metabolism -mainly respiration- as observed in figure 4A, which in turn decreases the consumption of organic acids and maintains a higher level of TA. As observed in the previous parameters, 1-MCP had no effect when the champa fruits were stored at 2°C. On the other hand, refrigeration at 2ºC and the use of 1-MCP maintained the highest TA values during the storage period in cape gooseberry fruits (Balaguera-López et al., 2015).
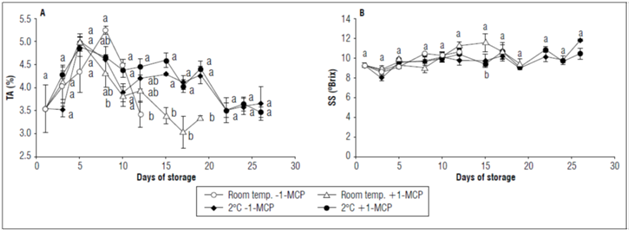
Figure 6. Effect of the postharvest application of 1-MCP and refrigeration on: A) Titratable acidity and B) Soluble solids of Champa fruits. The vertical bars in each mean indicate the standard error (n=4). Means followed by different letters in each measurement indicate significant differences according to the Tukey's test (P<0.05).
In general, there were no significant changes in SS during storage, but there was a slight increase in all treatments between the beginning and the end of this period. There were statistical differences (P≤0.05) only at 15 d, at which point the fruit with 1-MCP at room temperature had the highest SS values (9.4±0.5ºBrix), as compared to the refrigerated fruits, with or without 1-MCP applications (Fig. 6B). This result could have been due to the fact that the champa fruits at room temperature presented higher enzymatic activity related to carbohydrate metabolism, which increased soluble sugars. With refrigeration, the increase in SS was lower because the ripening process was delayed. The favorable effect of low temperatures on SS has also been found in tomato fruits (Getinet et al., 2008).
CONCLUSIONS
Ripening processes such as respiration, epidermis color and firmness of champa fruits were stimulated with the application of ethylene and delayed with the application of 1-MCP. Therefore, they are ethylene-dependent processes. Refrigeration at 2°C, with or without the addition of 1-MCP, maintained the fruits for 25 days because of a decrease in the respiration rate, weight loss, color index and loss of firmness. 1-MCP was effective only at room temperature, which is recommended for improving the postharvest conservation of champa fruits.















