INTRODUCTION
The potyviruses (genus Potyvirus family Potyviridae) is made up of 175 species (ICTV, 2020). This viral genus, non-persistently transmitted by more than 200 species of aphids, has a great capacity for adaptation to new environments and hosts and can infect monocotyledonous and dicotyledonous plants, including crops and wild plants (Nigam et al., 2019). Among the virus genera that infect plants, potyviruses are the most studied from the point of view of the functional characterization of their proteins, interaction with the host, evolution, taxonomy and diagnosis (Revers and Garcia, 2015). They have a flexuous particle that is 700-750 nm long, and their genome consists of a single strand of RNA with approximately 10,000 nucleotides with positive polarity. This potyvirus encodes a large polyprotein that undergoes proteolytic processing, providing essential functions for replication and movement. In the 5'3' direction, the proteins encoded by the potyviruses are P1, HC-Pro, P3N-PIPO, 6K1, CI, 6K2, VPg, NIa-Pro, NIb and CP. Among these proteins, NIb is an RNA dependent RNA polymerase or replicase whose function is viral genome replication (Hong and Hunt, 1996). The NIB genome sequence is well preserved and has been used for the design of universal primers for the detection of potyviruses (Ha et al., 2008; Zheng et al., 2010).
New species of potyvirus are identified every year. By 2019, 167 species had been reported (Nigam et al., 2019). By mid-2020, 175 species had already been identified (ICTV, 2020). The data confirmed that this genus is one of the more important groups of viruses that represent an economic risk and a threat to crops around the world.
Most diseases caused by potyviruses have symptoms such as mosaics, speckles, chlorotic rings or discoloration and deformation in foliage, flowers, fruits and stems, and necrosis of various tissues. Most cause severe delays in young plants and drastically reduce yields (Agrios, 2005). The severity of losses is proportional to the time the plant has been infected, i.e. how young the plant was when infected (Revers and Garcia, 2015).
Diagnosis of viral diseases can be addressed from different approaches, ranging from symptomatology in indicator plants, electron microscopy techniques, enzyme assays such as ELISA tests, and molecular methods including Polymerase Chain Reaction (PCR). The latter is the main route of identification and diagnosis in potyviruses (Díaz et al., 2010).
Molecular detection techniques can overcome many deficiencies of conventional assays, especially if they depend on PCR. PCR-based techniques are generally more specific and faster than conventional techniques (Mirmajlessi et al., 2015). One variant of PCR is RT-PCR, which is the molecular method for detecting RNA plant viruses. The design of primers for virus amplification is critical to the success in amplifying RNA targets through RT-PCR. Primers with a wide range of specificity should be designed from highly conserved genomic sequences. Different universal primers have been described for the detection of potyvirus (Langeveld et al., 1991; Pappu et al., 1993; Gibbs and Mackenzie, 1997; Chen et al., 2001; Ha et al., 2008; Zheng et al., 2010).
In Colombia, this potyvirus has been reported in crops such as the tree tomato (Ayala et al., 2010), papaya (Chaves-Bedoya and Ortiz-Rojas, 2015; Ortiz-Rojas and Chaves-Bedoya, 2017), potato (Riascos et al., 2017) and recently pepper (Rivera-Toro et al., 2021). Breeding programs need to know which viral agents, such as potyviruses, are present in the field. For the Department of Norte de Santander - Colombia, this information is very limited. This is the first study that looked for the presence of potyvirus infecting crop plants such as potatoes, corn, beans, peas and pumpkins in the provinces of Pamplona and Ocaña in Norte de Santander.The results suggest that, in both provinces, there was a broad presence of potyviruses infecting economically important crops; strategies for vector control must be improves to decrease viral incidence and economic losses in the region.
MATERIALS AND METHODS
Field samples. Plant samples from different crops were collected in the provinces of Pamplona and Ocaña in the months of November and December, 2018. The sampling methodology consisted of visually identifying symptoms in crops and collecting the necessary foliar tissue. The sampling included major crops of economic importance, such as corn (Zea mays), potatoes (Solanum tuberosum), beans (Phaseolus vulgaris), zucchini (Cucurbita pepo), tree tomatoes (Solanum betaceum), tomatoes (Solanum lycopersicum), onions (Allium fistolosum), peas (Pisum sativum), carrots (Daucus carota), peppers (Capsicum annuum), tobacco (Nicotiana tabacum), cassava (Manihot esculenta) and arracacha (Arracacia xanthorrhiza). The leaves of collected plants were placed in polystyrene containers with ice to keep them fresh and were taken to the research laboratory FITOBIOMOL at the Universidad Francisco de Paula Santander in the city of Cucuta. The leaves were stored in a Thermo Scientific Forma 8800 Series freezer at -70ºC until processing.
RNA extraction. Symptomatic leaves of different crop plants were used for the RNA extraction using the reagent TRIzol® (Invitrogen), starting with 100 mg of tissue according to the manufacturer's instructions. The RNA pellet was diluted in 75 µL of DEPC water. The RNA was quantified in a Genesys 10S UV-Vis spectrophotometer (Thermo Scientific) and stored in 15 µL of aliquots to prevent freezing, thawing, possible degradation of the entire sample. The RNA was used for reverse transcription and PCR reactions. A sample of papaya (Carica papaya) infected with the potyvirus PRSV was used as a positive control.
Primers. The positive-sense primer NIb2F and complementary NIb3R were used for PCR amplification. These primers were designed to amplify conserved sequences corresponding to the positions 7619-7641 and 7945-7968 of the Potato virus Y (PVY), accession No. AF522296. This pair of primers has been reported as the most effective in detecting potyvirus (Gibbs and Mackenzie, 1997; Thompson et al., 2003).
RT-PCR. The total RNA samples of each plant sample were used as a template to amplify the conserved NIB region of the potyviral genome. Reverse transcription was performed using the enzyme M-MuLV Reverse Transcriptase (New England) in a reaction volume of 25 µL, with 1 µL of the total RNA extract. Reverse transcription was performed at 48ºC for 45 min and then at 94ºC for 2 min to stop the reaction. The PCR amplification was initially performed as described Zheng et al. (2010). The PCR products were separated with electrophoresis at 100 V in 1.0% agarose gel and TAE buffer. The bands were visualized using GelRed®. The samples that were amplified to the expected size, as compared to the positive control, were assumed to be a successful detection of potyvirus.
Cloning and sequencing PCR products.Because of its economic importance and widespread presence in Norte de Santander, corn samples were used for further analysis. The PCR products of the expected size were purified using a QIAquick PCR Purification Kit (Quiagen) and cloned into the Invitrogen TOPOTM TA Cloning Kit vector following the manufacturer's instructions. Cloning was used to transform competent cells using a BIO-RAD MicroPulserTM electroporation device. Plasmid DNA was purified using a PureLink® Quick Plasmid purification kit (Invitrogen). Cloning was confirmed with restriction analysis and sequencing in triplicate. The consensus sequence in each case was used to identify the most related sequences in the GenBank (Altschul et al., 1997).
Sequence analysis. The alignment of nucleotides and deduced amino acid sequences was done with MegAlign. The manual editing of sequences was done using EditSeq (Lasergene, DNASTAR, Madison, WI). The phylogenetic relationships of the sequences were inferred by comparing the nucleotide sequence of Colombian SCVM isolates with sequences reported in the GenBank based on their nucleotide similarity and host. It has been reported that SCMV clusters in accordance with the host (Chaves-Bedoya and Ortiz-Rojas, 2012). The analyses were performed using the Maximum Likelihood test with 1000 iterations to estimate the confidence of the grouping. The GTR (General Time Reversible) model was used as the nucleotide replacement model. In cases where the resampling values were below 50%, the nodes were collapsed using TreeGraph 2 (Stover and Muller, 2010).
RESULTS AND DISCUSSION
Crop plant sample origin and preliminary assays
Rumex crispus, a weed present in some crop plots, was included. Weeds are considered alternative hosts that can act as a source and reservoir of viruses that could then infect nearby crop plants, contributing to the prevalence and temporary distribution of a virus in crops (Juarez et al., 2019). In total, 18 samples from the province of Pamplona and 22 samples from the province of Ocaña were collected (Tab. 1).
Table 1. Potyviral NIb amplification with RT-PCR in crop samples from two provinces of Norte de Santander - Colombia.
| Crop | Province | Samples | RT-PCR (+) | RT-PCR (-) |
|---|---|---|---|---|
| Allium fistolosum | Pamplona | 1 | 0 | 1 |
| Arracacia xanthorrhiza | Pamplona | 1 | 0 | 1 |
| Capsicum annuum | Ocaña | 3 | 0 | 3 |
| Cucurbita pepo | Pamplona | 3 | 3 | 0 |
| Cucurbita maxima | Ocaña | 2 | 2 | 0 |
| Daucus carota | Pamplona | 1 | 0 | 1 |
| Manihot esculenta | Ocaña | 1 | 0 | 1 |
| Nicotiana tabacum | Ocaña | 1 | 0 | 1 |
| Pisum sativum | Pamplona | 1 | 1 | 0 |
| Rumex crispus | Pamplona | 1 | 1 | 0 |
| Phaseolus vulgaris | Pamplona | 1 | 1 | 0 |
| Ocaña | 5 | 2 | 3 | |
| Solanum betaceum | Pamplona | 1 | 0 | 1 |
| Solanum licopersicum | Pamplona | 4 | 1 | 3 |
| Ocaña | 4 | 0 | 4 | |
| Solanum tuberosum | Pamplona | 2 | 2 | 0 |
| Zea mays | Pamplona | 2 | 1 | 1 |
| Ocaña | 6 | 3 | 3 | |
| Total | 40 | 17 | 23 | |
RNA extraction and RT-PCR
The oligonucleotides used in this study reproducibly amplified a band of 350 base pairs of a papaya sample infected with PRSV VR5 isolate (Chaves-Bedoya and Ortiz-Rojas 2015), a potyvirus from Villa del Rosario (Norte de Santander-Colombia), indicating the efficiency of the oligos. RNA was extracted from the plant samples indicated in table 1 as described in the methodology. Reverse transcription was performed with each of the RNAs using the NIB3R oligonucleotide.cDNAs were used to perform PCRs under these conditions. As shown in table 1, both in Ocaña and Pamplona, to the north and south of the department of Norte de Santander respectively, the presence of potyvirus was detected in crops such as Cucurbita pepo, Solanum lycopersicum, Zea corn, Cucurbita maxima, Phaseolus vulgaris, Pisum sativum, Solanum tuberosum and in the arvense Rumex crispus.
Amplicons of approximately 350 bp, as shown in figure 1, that matched the control were presumed to correspond to some potyvirus. A total of 17 out of 40 samples were positive for the RT-PCR for potyvirus (Tab. 1).

Figure 1. 1.0% agarose electrophoresis gel showing RT-PCR amplification of potyviruses NIb regions from crop plants in two provinces of Norte de Santander. 1, Tree tomato (Solanum betaceum); 2, zucchini (Cucurbita pepo); 3, corn (Zea mays); 4, tomato (Solanum lycopersicum); 5, potato (Solanum tuberosum); 6, pea (Pisum sativum); 7, bean (Phaseolus vulgaris); 8, corn (Zea mays); 9, pumpkin (Cucurbita maxima); (-) negative control; (+) positive control.
The PCR technique with potyvirus-specific primers is reliable and sometimes is the only laboratory procedure that leads to positive results. Recently, the successful case of detection of potyvirus by RT-PCR using specific oligonucleotides in Canna edulis Ker., was reported, while microscopy and serological techniques were negative in the same case(Betancourt et al., 2020).
In Colombia, studies have been carried out to characterize or identify potyvirus associated with different crops such as tree tomatoes and potatoes in the Department of Antioquia, which found the PYV (Ayala et al., 2010; Gutiérrez and Marín, 2018). On the other hand, SCMV (Sugarcane mosaic virus) has been reported as infecting unconventional hosts such as african oil palm (Elaeis guineensis) and achira (Canna edulis Ker.), both of which are reported in the Department of Nariño, in southwest Colombia (Morales et al., 2002; Betancourt et al., 2020). This is the first report of SCMV infecting corn in Norte de Santander although no information was found for the rest of Colombia.
In this study, the presence of potyvirus in a weed plant commonly known as “cow tongue” (Rumex crispus) was detected. The importance of these weeds and alternative hosts during the occurrence and spread of viral plant diseases is due to the fact that it is an integral part of viral epidemiology and can function as reservoirs of viruses and vectors throughout the year (Srinivasan et al., 2013). Alternative hosts serve as reservoirs of viral inoculum in live plant material that is present between the harvest of one crop and the planting of the next (Ranabhat et al., 2018).
The greatest effect from potyviruses was in Pamplona. Cucurbitaceae and corn were infected in both provinces, while tomato crops were affected in Ocaña. These results indicate a high presence of potyviruses infecting different crops. Under field conditions, plants can be infected with more than one virus, and the broad distribution of these plant pathogens in Norte de Santander indicates that efficient control measures are not being implemented to mitigate or prevent diseases. There is no availability of antiviral compounds to cure diseased plants, so early and accurate detection of plant-infecting viruses is essential. Plant diseases caused by viruses can be effectively controlled when management measures are applied at the beginning of the disease development or by planting virus-free crops (Joo-Jin et al., 2014).
Phylogenetic analysis of SCMV´s NIb
Among the positive results obtained with RT-PCR, corn samples were selected for confirmation with SCMV. The results of the BLAST (Basic Local Alignment Search Tool) analysis (Camacho et al., 2009) indicated that the sequences corresponded to the region approximately between position 7479-7774 of the complete genomic sequences of the Sugarcane mosaic virus (SCMV). Partial nucleotide sequences recovered from the NIb region of SCMV in Norte de Santander were reported in the GenBank with the access numbers MN607183 and MN607184. Table 2 shows the sequences used to build phylogenetic relationships of the new SCMV sequences with those already reported around the world.
Table 2. SCMV isolates used to build the phylogenetic tree. SCMV´s NIb sequences from Colombia are in bold.
| No | Accession | Host | Origin | Year | No | Accession | Host | Origin | Year |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MH093730 | Corn | Kenya | 2018 | 28 | JX047389 | Corn | China | 2017 |
| 2 | MH093728 | Corn | Kenya | 2018 | 29 | JX047386 | Corn | China | 2017 |
| 3 | MF467404 | Corn | Tanzania | 2020 | 30 | JX047383 | Corn | China | 2017 |
| 4 | MH093734 | Corn | Kenya | 2018 | 31 | JX047382 | Corn | China | 2017 |
| 5 | MF467398 | Corn | Tanzania | 2020 | 32 | JN021933 | Corn | China | 2011 |
| 6 | MF467397 | Corn | Tanzania | 2020 | 33 | JX047381 | Corn | China | 2017 |
| 7 | MF467394 | Corn | Tanzania | 2020 | 34 | JX047402 | Corn | China | 2017 |
| 8 | MH093727 | Corn | Kenya | 2018 | 35 | DQ647651 | Corn | Thailand | 2016 |
| 9 | MF467396 | Corn | Kenya | 2020 | 36 | KY006657 | Corn | Ecuador | 2016 |
| 11 | MF467401 | Corn | Tanzania | 2018 | 37 | KU886553 | Corn | China | 2018 |
| 12 | MF467400 | Corn | Tanzania | 2020 | 38 | JX047431 | Corn | China | 2017 |
| 13 | MF467399 | Corn | Tanzania | 2020 | 39 | JX047427 | Corn | China | 2017 |
| 14 | MF467395 | Corn | Tanzania | 2020 | 40 | JX047419 | Corn | China | 2017 |
| 15 | MF467393 | Corn | Tanzania | 2020 | 41 | JX047409 | Corn | China | 2017 |
| 16 | MK481076 | Corn | Kenya | 2020 | 42 | JX047408 | Corn | China | 2017 |
| 17 | MK481075 | Corn | Kenya | 2019 | 43 | KF744392 | Corn | Rwanda | 2014 |
| 18 | JX047415 | Corn | China | 2019 | 44 | MG932078 | Corn | Kenya | 2018 |
| 19 | JX047424 | Corn | China | 2017 | 45 | MH093720 | Corn | Kenya | 2018 |
| 20 | JX047412 | Corn | China | 2017 | 46 | MH205604 | Corn | Kenya | 2018 |
| 21 | JX047392 | Corn | China | 2017 | 47 | MG932077 | Corn | Kenya | 2018 |
| 22 | JX047416 | Corn | China | 2017 | 48 | MH795798 | Sugar cane | Nigeria | 2018 |
| 23 | JX047426 | Corn | China | 2017 | 49 | MH093723 | Corn | Kenya | 2018 |
| 24 | JX047401 | Corn | China | 2017 | 50 | MN607183 | Corn | Colombia | 2019 |
| 25 | JX047405 | Corn | China | 2017 | 51 | MN607184 | Corn | Colombia | 2019 |
| 26 | JX047387 | Corn | China | 2017 | 52 | EU091075 | Sugar Cane | México | 2011 |
| 27 | KT275937 | Papaya | Colombia | 2017 | -- | - | - | - | - |
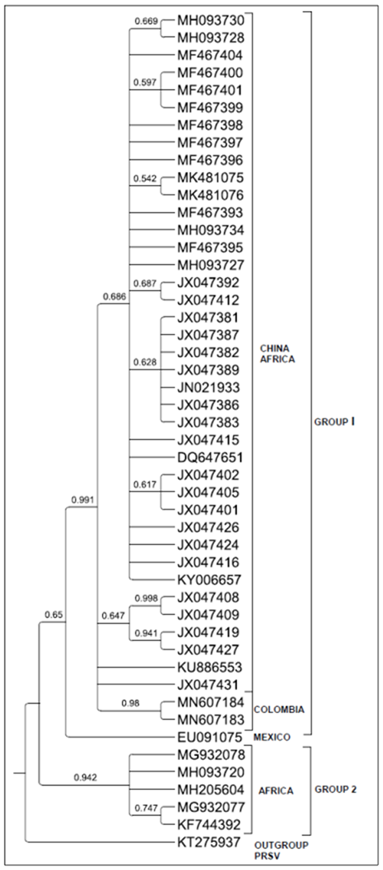
The phylogenetic tree (Fig. 2) was built including 50 partial sequences of the NIB region of SCMV recovered at GenBank (Clark et al., 2016). Most sequences were reported in China and Africa. In the phylogenetic tree, two groups are formed; the first group included isolates from Asia, Africa, Ecuador (KY006657) and Colombia (generated in this study). The second group had SCMV sequences reported in Africa.
The nucleotide distance matrix indicated that the percentages of similarity of the Colombian SCMV isolates with the African SCMV isolates that make up group II was 88.7%-90.1%, and the similarity was greater than 97% with the other isolates, including those from China and Africa. Among African SCMV sequences, the similarity was greater than 98%. The Colombian sequences had a similarity of 100%.
The potyvirus NIb protein is a RNA-dependent RNA polymerase (RdRP) that is absolutely required for potyviral genome replication and is a multifunctional protein. RdRPs are the only proteins encoded by all RNA viruses, making them ideal for evolutionary RNA virus analysis (Shen et al., 2020). The high similarity between the NIB sequences was due to the high degree of sequence conservation that resulted from the replicase function of this protein.
Because of their importance as pathogens, potyviruses have been studied more than other viruses (Revers and Garcia, 2015). However, in Colombia and the Department of Norte de Santander studies are scarce; much more remains to be explored.
Effective control and sustainable management of viral diseases in plants should be based on ecological and epidemiologically robust strategies that minimize the risk of epidemics and loss of associated crops (Ranabhat et al., 2018). Agricultural losses from pathogens can transform local economies and significantly reduce a food base resource for a community, affecting its well-being (Scholthof, 2007).
CONCLUSIONS
In Norte de Santander, potyviruses were detected in crops such as Cucurbita pepo, Solanum lycopersicum, Zea corn, Cucurbita maxima, Phaseolus vulgaris, Pisum sativum, Solanum tuberosum and in the arvense Rumex crispus.
The analysis of nucleotide sequences obtained from the corn samples confirmed the presence of the Sugarcane mosaic virus (SCMV); this is the first report of its presence in Norte de Santander.
Crops in Norte de Santander have precarious phytosanitary conditions because they are mostly infected with potyviruses. More studies are needed to identify other potyviral species present in the field in Norte de Santander, which would help develop appropriate and effective techniques that can treat diseases and achieve sustainable agriculture.