INTRODUCTION
High agricultural productivity is supported by fertilization with mineral nutrition (Taiz et al., 2017). World food production demands large amounts of inputs, among them fertilization represents 25-30% of the costs of fruit production (Gómez, 2012). Nutrient management is vital for growth, production, and quality, especially in economic and environmental sustainable crops (Mikkelsen, 2011). Inappropriate use of fertilizers prompts soil contamination, increases production costs and creates nutritional imbalances; and finally, itdecreases profitability and gains. Furthermore, in feijoa crops, deficiency of several elements can cause poor and low-quality production (Quintero, 2012). Mineral nutrients are necessary to carry out all metabolic reactions in the plant, and function as part of organic structure or as activators of enzymatic reactions (Taiz et al., 2017). Essential nutrient deficiencies lead to low yields because of alterations in the metabolic pathways where the element is involved, expressing such alterations in many cases as visible abnormalities (Epstein y Bloom, 2005).
In plants, symptoms of visual symptoms develop, representing the ultimate expression of a suboptimal supply of nutrients (de Bang et al., 2021). The recognition of these visible signs and symptoms is essential for performing the pertinent and necessary actions to mitigate impacts on profit. We should also point out that mineral deficiency is not the only concern for success with a crop. Other factors affect a crop such as an unsuitable environment, heavy metal toxicities, errors in the application of amendments, herbicides, or pesticides (Kumar y Sharma, 2013). For diagnosis, Gómez (2012) recommends considering the physiology and mobility of the element, the spatial distribution, and the location of the symptoms in the plant, as well as the senescence of the leaves. Symptoms must occur uniformly among the plants. If it is the case that a plant shows a pathological effect, the symptoms are visible only on isolated plants and can vary between them (Martínez, 2016).
Feijoa or pineapple guava (Acca sellowiana [O. Berg] Burret) and guava (Psidium guajava L.) both belong to the Myrtacea family (Fischer y Melgarejo, 2021). Feijoa, with a shrubby habit, is native to southern Brazil, Uruguay, western Paraguay, and northeastern Argentina (Fischer et al., 2020; Parra y Fischer, 2013). This species stands out due its adaptation to different climatic zones (Parra-Coronado et al., 2018). Similarly, the species can grow in poor soils, varying from sandy to clayey soil types (Fischer et al., 2020), but it shows its highest potential in deep alluvial soils that are rich in nutrients and organic matter, while sandy-loam to clay-loam promote root development of the plant (Quintero, 2014). In Colombia it is cultivated between 1,800-2,700 m a.s.l. (Parra-Coronado et al., 2019) with local temperatures between 13°C and 21°C (Fischer y Parra-Coronado, 2020). This fruit is desired in different national and international markets because of its organoleptic properties and the fact that it is involved in many transformed products, and therefore an important export opportunity for many Andean farmers (Fischer and Miranda, 2021). Additionally, this fruit has proved to have nutraceutical properties desirable to consumers (Fischer et al., 2003). In Colombia it is cultivated mainly by small farmers with a low level of technical assistance and technology (Quintero, 2012). In Colombia, the total production in 2018 was 2,765 t; however, the yield fluctuates widely from year to year, ranging from 7.6 to 10.6 t ha-1 between 2013 and 2018 (Agronet, 2021). The variability in yield is due to a poor understanding of aspects such as fertilization of this important crop.
Deficiencies of different macro and micronutrients impair specific physiological responses associated with their role in the metabolism of the plant and its yield (Marschner, 2012). Nitrogen (N) is after carbon the element required in greater quantity by plants; therefore, it is essential for growth and productivity (Taiz et al., 2017). Nitrogen plays a central role in plant metabolism as a component of proteins, nucleic acids, chlorophyll, coenzymes, phytohormones, and secondary metabolites (Kochhar and Gujral, 2020; Marschner, 2012). In feijoa, nitrogen deprivation reduces leaf quantity and general vigor of the plant (Quintero 2012). Phosphorus (P) is a structural element of nucleic acids with a function in energy transfer as a component of adenosine phosphates. It is also important for carbohydrate transfer in the leaf cells, as well as being used in large quantities to ensure yield (López-Arredondo et al., 2014). Kochhar and Gujral (2020) highlight osmoregulation as the main role of potassium (K) in the plant, important for cell extension and stomatal aperture. Potassium is required as a cofactor for more than 40 enzymes and is the main cation for the establishment of cell turgor and the maintenance of cell electroneutrality (Taiz et al., 2017). Moreover, this element promotes the sucrose loading mechanism and the movement of solutes driven by the massive flow within the plant (Marschner, 2012). Lambers and Oliveira (2019) mention that N has received more attention by physiologists, especially in higher altitude countries that are more characterized bygeologically young landscapes, where N is the main nutrient that limits plant productivity. However, as these last authors indicate, for most species in geologically ancient landscapes of the tropics and subtropics, for example in South and Central America, P is the main nutrient that limits productivity in many plants.
Crop competitiveness requires basic knowledge, such as understanding deficiencies and their physiological effects. Therefore, the main contribution of the present study is to identify and describe the symptoms and certain growth components related to fertilization deficiency in N, P, and K in young feijoa plants.
MATERIALS AND METHODS
Plants and establishment of the experiment
This research was carried out in field conditions at El Cortijo farm located at 4°55'9.59" N and 74°17'26.69" W in the municipality of San Francisco (Cundinamarca), at an altitude of 2,283 m a.s.l., a mean temperature of 13,2°C and relative humidity between 79 and 84%. The region's bimodal precipitation pattern has a mean annual of 1,400 mm. The plants were arranged in an area covered by a 6-gauge plastic film that served as a roof and protected the plants from rainfall. Inside, temperatures increased to a maximum of 22°C at noon.
The plant variety used was clone 41 ('Quimba'). A total of 72 plants 9 months old were used from the farm's nursery with a mean plant height of 125 cm. The plants were transplanted into plastic bags with a capacity of 10 kg that were filled with pale gray quartz sand with a grain size of 1.2 mm and CE of 0,011 dS m-1. The adaptation period last 2 months (June 30 to August 31). On August 31, treatments were begun until December 11, 2016. The plants were placed in three rows 0.25 m and 0.4 m between plants and rows, respectively.
Evaluations were performed every 15 d from day 39 following the start of treatments for quantitative variables: apical growth, production of apical shoots, production of basal shoots, and leaf chlorophyll content. CCI units were measured with a portable chlorophyll meter CCM-200(Opti Sciences, Tynsgboro, MA, USA). The procedure was done in triplicate for each replica, for leaves of different height level, high, medium, and low. The growth rate (cm d-1) was determined based on an article published by Hunt (1990); the author presents this variable as the simplest in growth analysis, determining only the change in size per unit of time. In this experiment the procedure was applied to the change in the length of lateral shoots per unit of time. A description of the symptoms manifested by the plants was developed with a photographic record.Symptoms determined for each deficiency expressed included chlorosis, meristem wilt, marginal necrosis, and growth retardation. Photography was realized every 8 d starting 20 d after the start of treatments.
Modified Hoagland and Arnon solutions were developed for each treatment to evaluate the effect of the treatments (Martínez et al., 2009), withholding a single element in each application (Tab. 1). The doses in table 1 were adjusted based on guava extraction levels and using foliar analyses reported in feijoa, adjusted for this experiment (Natale et al., 2009; Fischer et al., 2003). The mixture was prepared for concentrated solutions of 1 liter and was applied twice a week together with irrigation. A physicochemical analysis of the irrigation water was done to validate its suitability for the study. The compounds used were calcium chloride (CaCl2), dipotassium phosphate (K2HPO4), ammonium phosphate ((NH4)3PO4), calcium nitrate (Ca(NO3)2), potassium sulfate (K2SO4), magnesium sulphate (MgSO4), potassium nitrate (KNO3), urea (CH4N2O), sodium borate (Na2B4O7·10H2O), EDTA chelated compounds for minors (Fe, Cu, Mn, Zn, Mo, Co).
Data analysis
A completely randomized design was established, determining a feijoa plant as an experimental unit. Five treatments and nine repetitions (each repetition consisted of one plant) were established. Five non-destructive and one destructive sampling were carried out at the end of the experiment. Data were analyzed with an ANOVA and the Tukey test with a significance of 5% using the SAS (Statistical Analysis System), v. 9.2, program.
RESULTS AND DISCUSSION
Symptoms registered
After 60 d, we observed that among all treatments, the N-deficient plant had the most delayed development (Fig. 1A-E), confirmed by other studies using the method of the missing element in nutrition (Martínez, 2016). In young plants of guava (another Myrtaceae) Dussán et al. (2016) find that the -N treatment reduce the number of leaves and dry mass accumulation by all plant structures, becoming the most limiting element in the vegetative phase of these plants.
Nitrogen
Although Duarte and Paull (2015) state that feijoa is a slow-growing tree with a relatively low N requirement in relation to potassium and phosphorus, the N-deficient plants show less vigor with respect to the control. This was expected by the amount of N required by the plant that occupied between 1% and 5% of its dry weight (Marschner, 2012). Lower vigor confirms that N is the mineral element that plants require in greater quantities and its deficiency inhibits the regular growth of plants (Taiz et al., 2017). We observed increased initial susceptibility to host algae from the study site (Fig. 2A). Likewise, we saw widespread chlorosis in old leaves (Fig. 3A) (Gómez, 2012; Epstein y Bloom, 2005) and in addition a slight activation of growth zones (Fig. 3B) and overall pale green coloration (Fig. 3C) (Kochhar and Gujral, 2020), probably generated by the loss of chlorophyll that requires N as a basic constituent (Scheible et al., 2004). Apical damage was also seen in young leaves (Fig. 3D), characterized by brown coloration at the apex of the leaf (Röber y Schacht, 2008). Furthermore, dark purple leaf blades were observed (Fig. 2B). Probably, the carbohydrates not spent in nitrogen metabolism were used in the synthesis of anthocyanins, which led to the accumulation of this pigment and the purple coloration of leaves (Taiz et al., 2017). In eucalyptus trees, N deficiency, in addition to generating pale green and chlorotic leaves, with a reddish margin and in some cases, reddish spots are distributed throughout the leaf, the oldest leaves turned completely red (Silva-Cardoso et al., 2014). This could suggest the accumulation of anthocyanins in N-deficient plants (Kochhar and Gujral, 2020) within the Myrtaceae family.

Photos: S. Buitrago.
Figure 2. Leaves -N: A, algae host; B, old -N leaves with generalized purple coloration.
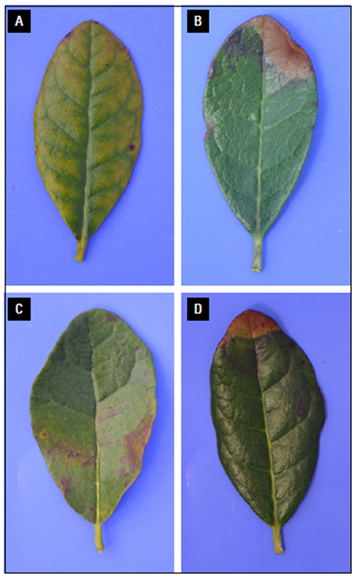
Photos: S. Buitrago.
Figure 3. Leaves -N: A, general chlorosis on old leaf; B, old leaf with brown apical spot; C, marginal necrosis and pale green coloration; D, old leaf with apical damage.
In agreement with Epstein and Bloom (2005) and Rajendran et al. (2009), the detriment of the leaves green color intensity from the lower to the upper strata was observed. This green discoloration that begins at the base of the plant is because N deficiency usually begins in older leaves as the N is re-mobilized to younger leaves (Marschner, 2012).
Phosphorus
The symptoms associated with this element were not clear until two months after the initiation of treatments. The first observations involved malformation of new leaves with chlorosis in their distal area in the middle and upper third of the plant (Epstein y Bloom, 2005) (Fig. 4A), and gray-black-purple necrosis in the central vein of the leaf (Fig. 4B), also observed by Martínez et al. (2009) in cape gooseberry leaves with this deficiency, as well as necrotic spots on the distal foliar margin (Fig. 5A). Necrotic spots were also found in concentric rings on ancient leaves (Fig. 5B).

Photos: S. Buitrago.
Figure 4. Leaves -P: A, symmetric malformation in leaves; B, central part along central vein gray-black-purple in old leaves.
Photos: S. Buitrago.
Figure 5. Leaves -P: A, necrotic spots in the apical marginal area; B, necrotic spots on old plant leaves.
According to Taiz et al. (2017) and Kochhar and Gujral (2020), the characteristic symptoms of phosphorus deficiency include dark green leaf coloration, and the leaves may be malformed (Fig. 4A) and contain small areas of dead tissue (necrotic patches) (Fig. 5A and B). Moreover, as in the case of nitrogen deficiency (Fig. 2B), some species may produce an excess of anthocyanins in case of phosphorus deficiency, giving the leaves a slightly violet color (Fig. 5B) (Close and Beadle, 2003; Chen et al., 2018; Peng et al., 2020). Although the reduction in growth variables does not coincide with that indicated by Pallardy (2008), our experiment confirmed what is reported by Röber and Schacht (2008) and Rajendran et al. (2009). On the vine, P-deficient leaves show a dark-green color because foliar expansion is inhibited to a greater extent than chlorophyll formation (Marschner, 2012). In rice, Gamuyao et al. (2012) published an important study regarding the Phosphorus Starvation Tolerance 1 (PSTOL1) gene, although its role in root elongation is widely discussed, the direct effects on the foliage are summarized as an influence on phosphorylation in the thylakoid. Therefore, malformations observed in our experiment may underlie a lack of necessary energy to successfully complete mitosis, thus limiting the correct formation of photosynthetic organs.
Potassium
The physiological disorder caused by the lack of this element did not affect plant vigor (Fig. 1D). Crinkling was found in new leaves (Fig. 6A). In the basal shoots, damage was found in the last third of the leaves (Fig. 6B). On the ancient leaves the margin turned brown and then necrotic (Fig. 7A). Gómez (2012) and Röber and Schacht (2008) show the same effect in tamarillo and describe it with necrotic margins bent up or down. New leaves became concave (epinasty) (Fig. 7A), or with slight chlorosis in the middle part of the leaf (Fig. 7B), perhaps caused by a lack of turgor (Martínez, 2016) and necrosis in the margin (Fig. 7C) and at the tips (Fig. 7D), as observed in citrus (Rajendran et al., 2009).

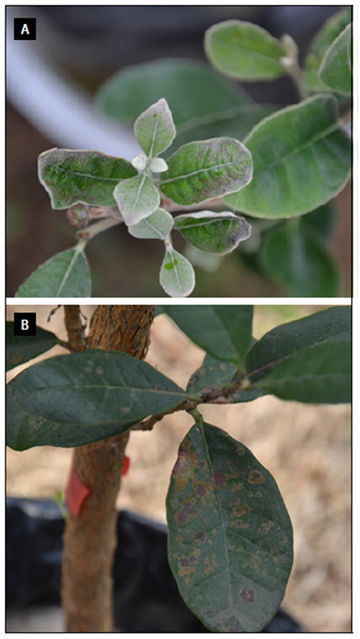
Photos: S. Buitrago.
Figure 6. Leaves -K: A, epinasty in fully unfolded young leaves; B, damage to the last third of leaves.

Photos: S. Buitrago.
Figure 7. Leaves -K: A, mature leaf with brown leaf margin; B, young leaf with symptoms of chlorosis in the second middle of the leaf; C, young leaf with marginal chlorosis; D, young leaf with necrotic distal area.
Similar symptoms are reported by Pallardy (2008) as follow: potassium deficiency in woody plants is associated with marginal chlorosis in old leaves, followed by necrotic points that coalesce. These symptoms are recorded in woody species such as cotton (Gerardeaux et al., 2010), citrus (Zekri and Obreza, 2013), apple (Valdebenito-Sanhueza et al., 2008) and mango (Prado et al., 2012). In avocado, K deficiency (Fig. 7A and D) clearly coincides with reports made by Novoa et al. (2018), with apical necrosis in old leaves.
Marschner (2012) describes K-deficient leaves that become chlorotic and necrotic, depending on the light intensity they receive. In addition, this author highlights the importance of K for the accumulation of solutes creating the internal osmotic potential necessary for leaf turgor that may explain the epinasty and lack of turgor in feijoa leaves (Röber and Schacht, 2008). In figure 6B, chlorosis was observed in basal shoots that may be associated with interruptions in the movement of fructose inside the plant, an event that would affect the development of basal shoots (Taiz et al., 2017).
Effect on growth variables
The apical growth of plants has not been significantly affected by the lack of these three macronutrients (Tab. 2). However, Duarte and Paull (2015) argue that feijoa is a slow-growing plant, therefore it has a relatively low nitrogen requirement compared to potassium and phosphorus. Especially for the case of N, the tendency of a reduction in terminal growth is known (Kochhar and Gujral, 2020). This deficiency is characterized as a delay in its development emphasized by the slower growth of the plant (Epstein and Bloom, 2005).
Table 2. Variables of growth and chlorophyll content in feijoa seedlings, after 102 d from the start of the experiment, with the missing elements of -N, -P and -K, control (complete nutrition) and only water.
| Variable | - Nitrogen | - Phosphorus | - Potassium | Control | Only H2O |
|---|---|---|---|---|---|
| Apical growth (cm) | 4.72±3.96 ab | 6.33±2.97 ab | 6.89± ab | 9.14±4.53a | 1.94±4.91 b |
| Number of apical shoots | 3.22±2.68 bc | 9.00±2.74 ab | 8.77±6.22 ab | 12.89±5.46 a | 1.00±2.00 c |
| Number of basal shoots | 0.11±0.33 b | 1.78±1.56 ab | 2.44±2.79 a | 1.56±1.59 ab | 0.11±0.33 b |
| Chlorophyll content index (CCI) | 72.25±19.57 b | 76.59±11.12 ab | 72.53±13.21 ab | 86.67±11.24 a | 67.01±13.50 b |
| Growth rate (cm d-1) | 0.115±0.08 ab | 0.055±0.10 bcd | 0.004±0.01 d | 0.161±0.11 a | 0.008±0.02 d |
Means with different letters, in the same row, indicate significant difference according to the Tukey test (P≤0.05); ± indicates the standard deviation.
Lambers and Oliveira (2019) describe plant growth as the result of an interactions between the processes of photosynthesis, respiration, long-range transport, water-plant relationships, and mineral nutrition.
This greater impact on the apical growth of N-deficient plants was significantly reflected in the number of apical shoots developed (Tab. 2). The number of terminal shoots was 3.2, only a quarter of those produced by the control, a result that highlights the low investment of these plants -N in new growth and photosynthetically active tissues (Lambers and Oliveira, 2019).
Likewise, in the production of the basal shoots, the N deficiency resulted in a number as low as 0.11, the same as the plants that were irrigated only with water, and this was significantly lower than the plants with -K deficiency (Tab. 2).This minimal production of basal shoots in plants with -N deficiency, is likely to be explained because this element is translocated from the most mature (and low) to youngest and apical leaves (Marschner, 2012). This effect was more drastic in plants with -N deficiency than those with -K deficiency, also apically translocated in the plant. Furthermore, this result confirmed that N is the nutrient most required by the plant, contraDuarte and Paull (2015); and its deficiency quickly inhibits its growth (Taiz et al., 2017; Dussán et al., 2016). This fact may be especially important in adult feijoa crops in which pruning represents an important agricultural task in the creation of new productive shoots (Schotsmans et al., 2011).
Chlorophyll content is not only accepted as an indirect measure of nitrogen content (Moran et al., 2000), due to its fundamental role in these components structure (Kochhar and Gujral, 2020), but also is accepted as a measure of plant stress (Coste et al., 2010). The chlorophyll content results are significantly lower than control in nitrogen deficient plants. This is in line with the lower growth and number of apical and basal shoots (Tab. 2). Marschner (2012) points out that the lack of N can trigger a decomposition of nucleic acids and proteins in the leaves, causing foliar senescence that generates decomposition of rubisco and, therefore, a decrease in the maximum photosynthetic capacity of the plant, finally inhibiting growth. Moreover, N translocation to younger leaves may cause this low nitrogen content in the remaining leaves (Marschner, 2012). Leaf chlorosis is closely related to nitrogen supply and iron absorption, altering the concentration of leaf chlorophyll (Granja y Covarrubias, 2018). Although the low chlorophyll content is a symptom associated with N deficiency plant, this variable is not merely limited to N deprivation. The chlorophyll content is also significantly affected in P and K deficient plants (Kalaji et al., 2017).
Growth rate relates the growth of lateral shoots per unit of time (Hunt, 1990). This variable is significantly lower in P-deficient plants that can be related to the rapid decrease in photosynthetic rates (Orduz-Ríos et al., 2020), because many intermediate stages for carbon fixation involve sugar phosphates (Maathuis, 2009). Hurtado et al. (2019) observe slow shoot growth in juvenile avocado plants with -P deficiency, while Dussán et al. (2016) record a serious affectation of the foliar area in plants with -P deficiency in guava. Likewise, growth rate is significantly lower in plants with -K deficiency, which could be explained, first, by translocation altered pattern of photo-assimilates to the new growing shoots (Marschner, 2012) and, second, possibly by the impairment in the photosynthesis process caused by the deficiency of this nutrient, due to its role in stomatal regulation, and finally, in its role in stimulating stem elongation by gibberellic acid which is also K dependent (Marschner, 2012). This effect was not seen during the first days of our experiment. After 88 d of treatment, growth showed a great reduction, probably caused by the mobility of K being highly mobile. This element could have been transferred to the growth zones in the first days of the experiment; however, after 88 d of deficiency the impact on the creation of new biomass was significant and drastically limited. On the other hand, in the three-month-old guava seedlings, the height of the plants was not significantly decreased between the control and deficient in potassium treatments during 98 d of sampling (Dussán et al., 2016). This suggests a slow sensitivity to the effects of potassium deficiency.
In a brief approximation, the symptoms associated with N, P, and K deficiency in nine-month-old feijoa seedlings were identified and described. There is high variability between the symptoms found, and the times of occurrence of each one. For the elements N, P, and K, a time of more than 60 d was required after initiating treatments for the expression of symptoms. Thus, new studies on physiology and nutrition in feijoa are required, with insights for precise and timely tools to avoid production losses associated with inadequate fertilization.
CONCLUSIONS
The ultimate expression of a suboptimal supply of nutrients are the visual symptoms. Their recognition is essential to perform the pertinent and necessary actions towards mitigating any impact on profit. In feijoa, research with this approach had not been carried out in Colombia before. For this reason, in young feijoa plants symptoms of poor fertilization in N, P, and K, applying the missing element method, was realized. The greatest effect on the plants was due to a lack of nitrogen because of reduced growth and a global pale green coloration. But also, P and K deficiencies showed symptoms and growth characteristics that were altered during the development of the plants. While -P plants showed foliar malformations and necrosis, -K plants exhibited the brown margin in old leaves and then necrosis, while young leaves expressed a lack of turgor. The described and thorough photographic record presented here shows symptoms that are important for recognizing these nutrient deficiencies in the field.





![Mathematical models for describing growth in peach (Prunus persica [L.] Batsch.) fruit cv. Dorado](/img/en/next.gif)










