INTRODUCTION
The almond tree, (Prunus dulcis Miller), is a species with great genetic variability; the fruit is a drupe, with its outer part formed by the pericarp and the mesocarp. Prunus amygdalus is now recognized as the result of natural hybridizations involving several wild species (Prunus bucharica and Prunus kuramica) that still exist in central and southwestern Asia (Grasselly and Crossa-Raynaud, 1980). The almond is rich in vitamins and minerals and is considered a good source of vitamin E (tocopherols), riboflavin, calcium, magnesium, phosphorus, potassium, zinc, copper and manganese (Rodushkin et al., 2008; Ballhorn, 2011). Almonds also contain a wide variety of phenolic compounds, mainly proanthocyanidins, flavonoids and phenolic acids (Hayes et al., 2016) that are predominantly found in the skin and are responsible for their antioxidant properties (Mandalari et al., 2010). Phytosterols are also found in significant quantities (~270 mg/100 g) in sunflower-almond grains, the predominant type being β-sitosterol (Fernández-Cuesta et al., 2012; Alasalvar and Bolling, 2015; Forcadaet al., 2015). Other compounds with an important characteristic in bitter almonds include glucoside amygdalin, responsible for the bitter taste, whose proportion in the grain is 2 to 4% (Ibar, 1985).
The main use of sweet almonds is human consumption, either alone or as part of other products (Bainbridge, 1996). Other uses include flour for pastries, fatty acids to produce margarine, and edible oil, obtained from bitter almonds after eliminating hydrocyanic acid (Abd Aal et al., 1987). Almonds are also used as ingredients in ice cream and various cooking recipes. The fatty acids in almonds serve as caking preventives and external preservatives in extruded snacks (Kobayashi and Hisamatsu, 1978).
The bitter taste of almonds is due to glucoside amygdalin (McCarty et al., 1952; Conn, 1980; Polesello and Rizzolo, 1989; Frehner et al., 1990; Sánchez-Pérez et al., 2008; Sánchez-Pérez et al., 2019); studies have shown that the sweet or bitter taste of almonds is a monogenic characteristic, where the bitter flavor is a homozygous recessive (Heppner, 1923; Heppner 1926; Dicenta and García, 1993; Vargas et al., 2001;Thodberg et al., 2018). However, it has been shown that the sweet or sour taste of almonds is characteristic of the different varieties and is not influenced by the type of pollen that pollinated the flower (Dicenta et al., 2002). Cyanogenic compounds can produce hydrocyanic acid under certain conditions, and, in the presence of specific enzymes, cyanogenic glycosides are structurally very similar, in some cases changing the position of the radicals.
The toxicity of cyanogenic glycosides and their derivatives depends on the release of hydrogen cyanide (FAO and WHO, 2012). The primary action of hydrocyanic acid in a person's body is to inhibit cytochrome oxidase, which blocks cellular respiration. The lethal dose of hydrocyanic acid in humans is 0.5 to 3.5 mg kg-1 of body weight in a single dose (Blum, 2010; Borron and Baud, 2012). A person with mild cyanide poisoning suffers headache, nausea and weakness as the result of oxygen deprivation, conditioned on the concentration of the compound and the exposure period. When it is ingested small amounts of cyanogenic compounds, the most common route for detoxification is the conversion of hydrocyanic acid to thiocyanates in the liver and kidneys, which are subsequently excreted in the urine (Chaouali et al., 2013; Abraham, et al., 2016); importantly, the cyanide concentration is higher in erythrocytes than in plasma. Studies have shown that the cyanide level in different human tissues in a fatal case of HCN poisoning is 0.03 gastric content, 0.50 blood, 0.03 liver, 0.11 kidney, 0.07 brain, and 0.20 urine (mg/100 g) (EPA, 1990); toxic levels of cyanogenic glycosides are estimated based on the amount of free cyanide generated after hydrolysis (EFSA, 2007). However, a level of cyanide up to (10 mg L-1) has been reported as safe for cassava flour (FAO and WHO, 2012). The lack of quantitative toxicological tests and epidemiological information makes it difficult to establish a safe intake level for cyanogenic glycosides in many foods. The objective of this research was to evaluate toxicity levels of some cyanogenic compounds, considering their conversion to cyanhydric acid in almonds for consumption and industrial uses.
MATERIALS AND METHODS
Vegetal material
Table 1: almond varieties collected on the experimental farm "Tres Caminos" of the Center for Soil Science and Safe Applied Biology (CEBAS), of the Higher Council for Scientific Research (CSIC). The farm is in Santomera (Murcia), at 130 m a.s.l., with very hot summers and mild winters and with minimum temperatures that do not usually drop below (0-4°C). The trees, of different ages, have localized irrigation and different planting frames, depending on the case.
Table 1. Almond varieties and theoretical flavor in each harvest year.
| Sweet | ‘Desmayo´ ´Largueta’, ‘Del Cid’, ‘Atocha’, ‘Ferragnès’, ‘Peraleja’, ‘Primorskii’, ‘Marcona’, ‘Ramillete’, ‘Ferraduel’, ‘Achaak’, ‘Planeta’, ‘Bonita’, ‘Colorada’, ‘Carretas’, ‘La Mona’, ‘Tioga’, ‘Titan’, ‘CEBAS’, ‘Pajarera’, ‘Rumbeta’ |
| Slightly bitter | ‘Garrigues’, ‘Genco’, ‘Tuono’ |
| Bitter | ‘S3060’, ‘S3062’, ‘S3076’, ‘S3108’, ‘S3112’, ‘S3126’ |
Collection, conditioning and conservation of the sample
The almonds were taken from the trees at full maturity when the mesocarp was completely open; each sample was made up of 50 almonds that were randomly collected. The almonds were stripped of the mesocarp and placed in mesh bags, duly labeled, and transported to the laboratory. The samples were lyophilized (Telstar 2000 lyophilizer), at a pressure of 4·10-2 mbar and a temperature between -79 and -82°C, then they were kept at -18°C until subsequent analyses, evaluated two years in a row.
Bromatological characterization
The seeds were manually extracted. The characterization of the almonds was carried out using the techniques of proximal analysis: moisture with method 930.15 (AOAC, 1990); determination of ether extract with method 920.39 (AOAC, 1990); ash determination with method 942.04 (AOAC, 1990); crude fiber determination with method 962.09 (AOAC, 1990); crude protein determination with method 955.04 (AOAC, 1990); and fat determination with Colombian technical standard - NTC 336 (ICONTEC, 2002) (EFSA, 2007).
Thermophysical analysis
The thermophysical properties determined for the almond seeds were thermal conductivity, thermal diffusivity, specific heat, and density. These were estimated according to the bromatological composition, using mathematical models based on temperature, in a range from - 40 to 150ºC (Choi and Okos, 1986). The models are presented in table 2. The results were compared with those of Deproter® v. 2 software (2012).
Table 2. Models used to determine thermophysical properties in the almonds.
| Thermal conductivity (k) |
|
| Density (ρ) |
|
| Thermaldiffusivity (α) |
|
| Specific heat (C p ) |
|
Chemical analysis of cyaniogenic compounds
The cyaniogenic compounds of the almonds were determined with microdiffusion and high-performance liquid chromatography (HPLC) (Fig. 1). The chromatographic conditions included: Symmetry C18 column, eluent acetonitrile: water (80: 20), flow rate 1.3 mL min-1, and photometric detector at 218 nm. The coefficient of variation obtained in the chromatography, as a measure of the reproducibility of the method, was 2.3% at concentration levels 100 times above the detection limit and 22% at concentration levels close to the detection limit. The detection limit for amygdalin was 0.387 mg/100 g and 0.136 mg/100 g for prunasin.
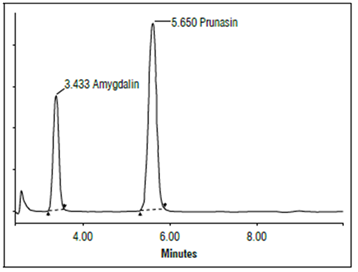
Source: Arrázola et al. (2015).
Figure 1. Chromatogram of a standard mixture of amygdalin (3.4 min) and prunasin (5.7 min) obtained using a Symmetry C18 column.
Microdiffusion method
The hydrolysis of the glucoside (first stage) was carried out as follows: generation of hydrocyanic acid with acid hydrolysis (Haque and Bradbury, 2002) using autoenzymatic methods but controlling and slowing down the process of generating hydrocyanic acid by adding reagents that inhibit hydrolysis. Once the hydrocyanic acid was generated with hydrolysis, it diffused into the microdiffusion cell designed for this purpose and was absorbed in a concentrated 0.2N NaOH solution placed inside the device where the hydrolysis was carried out. For the quantitative analysis, concentrations greater than 10 mg L-1 were created with gravimetry using silver nitrate and a rhodanine solution as an indicator (Bark and Higson, 1963). For the determination with picrate, a container like the one in figure 2 (Egan et al., 1998) was used, where a support was placed that contained the enzyme at one end (own) necessary for the hydrolysis of the cyanogenic compound and strip of paper impregnated with sodium picrate at the other end (yellow in color), which turned orange when cyanide was released. This method has also been used for quantitative determination, extracting the compound formed by the Guignard reaction from the paper strip and measuring the absorbance of the extract at 520 nm (Lucas and Sotelo, 1984). Egan et al. (1998) developed a technique that makes it possible to measure absorbance in the solid state, that is, in the strip of paper itself.
Hydrolysis and microdiffusion of cyanide in almonds
0.2-0.4 (±0.0001) g of precisely weighed freeze-dried bitter or slightly bitter almonds were placed in the reactor. The collector vial placed inside the reactor, as detailed in figure 2, with 1.0 mL of 0.2 M NaOH solution was available, and 4 mL of phosphate buffer pH = 5.5 was added to the sample to adjust the pH. The reactor was immediately closed. It was kept in a water bath at 35ºC for 24 h; afterwards, the reactor was removed from the bath and allowed to cool. Then, the reactor was opened, and the cyanide was collected from the collecting vial for its determination either with colorimetry or titration gravimetric, according to the concentration. The extraction performance was evaluated as follows: A sample was prepared with 5 mL of CN- standard with 1.0 mL of 0.2 M NaOH (1 mg L-1) in the collecting vial and 0.7 mL of phosphoric acid to adjust the pH to 5.5. The microdiffusion procedure was applied followed by the colorimetric determination, and the yield was determined, which turned out to be 94%.
Colorimetric determination of total cyanide (low concentrations of CN-)
The alkaline solution from the diffusion collector was quantitatively transferred to a 25 mL flask, with a dropper, washing the collector with 4 mL of NaOH (measured with a pipette and poured into a 50 mL beaker) in three portions, which were added to the flask. 5 mL of the KH2PO4 solution were added, immediately followed with 0.5 mL of chloramine. It was allowed to stand for 1 min. 1 mL of barbituric acid was added after 20 min; the absorbance was measured at 580 nm, against a blank prepared with 5 mL 0.2 M NaOH + 5.0 mL KH2PO2 + 0.5 mL of chloramine + 1.0 mL of barbiturate. The determination was made by comparing the absorbance of a standard prepared from 5 mL of CN in 0.2 M NaOH (1 mg L-1) + 5.0 mL KH2PO4 + 0.5 mL chloramine + 1.0 mL barbituric acid, also brought to 25.0 mL, which gave an absorbance versus the blank of (0.810-0.850). If the absorbance of a test sample is <0.020; it must be calculated with the following equation (1) since the cyanide concentration is below the detection limit
where, C M was sample concentration, V f was final volume, P i was sample weight (g), A M was sample absorbance, A p was standard absorbance, C p = Cyanide was standard concentration (mg L-1), and f = Factor due to 94% recovery.
The cyanide concentration was calculated in mg/100g of dry sample by comparing the absorbance of the sample (A
M
) with the absorbance of the standard (A
p
) of 1 mg L-1 of cyanide from which 5 mL were taken and taken to a volume of 25 mL, with which the concentration of the standard (C
p
) was 0.2 mg L-1, thus:
The extraction yield was 94%, which indicated that the factor:
Gravimetric titration of cyanides (for high concentrations of CN-)
The alkaline solution of CN- from the collector was quantitatively transferred to a 100 mL beaker, with a dropper, washing it and combining the washings with the first transfer, with (3) three portions of H2O of 1-2 mL. It was diluted to 50 mL with water, and 1 drop of indicator was added. AgNO3 titrant reagent was slowly added (especially near the turn) with magnetic stirring, until it turned from yellow to reddish. The grams of AgNO3 spent in the titration were calculated with the weight difference of the flask containing said solution before starting the titration and after the end point; the reaction was as follows (3):
A blank was made with 1 mL 0.2 M NaOH + H2O up to 50 mL + 1 drops of indicator (4).
Flour elaboration
The almond flour elaboration process was carried out to use them in the different analyses in this study to quantify the level of toxicity and products; the necessary unit operations were carried out, such as seed suitability, peeling, grinding, and classification by particle size; 50 whole units were left to obtain candied almonds according to Arrázola et al. (2015).
Manufacture of candied almonds
Although the consumption of dry products including almonds is growing, this study considered the process for candied almonds from the seeds of almond trees, for which the manufacturer's grading drum was used (Fedeacero, model WQA 2011, Bogota, DC). The kernels were precoated with powdered sucrose syrup and gelatin. The ratio of the precoat to the cores was 5:1 p/w. The procedure consisted of adding the kernels (almond seeds) to the crushing drum, adjusting the drum inclination to 45° and the revolutions to 30 rpm (Andréo et al., 2007). Then, the precoating solution (syrup) was slowly added with consequent drying of the almonds with hot air (±45°C). The coating solution was added gradually with subsequent drying by air. Finally, the coating powder (sugar and starch) was added, and the cores were dried at the previous temperature. Then, the thickening and smoothing of the nuclei were carried out. The former was carried out with a solution of sucrose with dyes at a ratio of 0.9: 1 p/p, times the weight of the nuclei, and the smoothing of the nuclei was carried out using USP syrup with powdered sugar dissolved at a ratio of 1.9: 1 p/w, times the weight of the cores. Both stages were carried out under the same procedure described above (pre-coating), except for the application of coating powders and a higher grading drum inclination (Arrázola et al., 2015).
Statistical analysis
An analysis of variance (ANOVA) and Tukey's HSD test were performed, with a significance level of 5% for the results obtained in the quantification of amygdalin, prunasin and hydrocyanic acid to determine the influence of the levels of cyanogenic compounds converted to hydrocyanic acid. In addition, a correlation was made from the R-Pearson test between each of the thermophysical parameters. The correlation was considered highly significant at the 0.01 level (two-sided). The data were processed using Minitab Inc.® version 16.0.
RESULTS AND DISCUSSION
Industrial uses and components
An alternative for handling and conserving almonds is their confit with very thin films, whose coating provides about 12 months, guaranteeing their chemical and nutritional composition. In obtaining flour, the particle diameter of almonds presented sizes between 500 and 250 μm, with 70% sieve passing through the 250 μm. The fat and moisture content are factors to consider in the treatment of almonds potentially destined for industrial processing because they affect the conservation of raw material and play a role in the technological use of almonds although this was not an objective in this study. However, the high percentage of crude fat, with percentages of 51%, offers the opportunity to extract oil for the preparation of pharmaceutical and cosmetic products. These results are similar to industrially obtained products, used as raw materials (Arrázola et al., 2013; Delgado-Tobon et al., 2018). Table 3 shows the bromatological results:
Table 3. Compositional analysis of almonds (mixtures) (Prunus dulcis Miller).
| Composition | Percentage (%) |
|---|---|
| Moisture | 17.18±02 |
| Fat | 51.00±01 |
| Ash | 5.00±03 |
| Protein | 15.60±05 |
| Fiber | 9.50±01 |
| Carbohydrate | 1.72±0.02 |
Source: Arrázola et al. (2013)
For the candied almonds, the almonds were toasted and coated with syrups and food additives (color). Candied almonds coated in various colors were obtained, where the thermophysical characteristics of the almonds were determined as one of the objectives given the need-to-know the thermophysical values.
The diffusivity, thermal conductivity, specific heat and density of fresh almonds was 1.13·10-7 m2 s-1, 0.32 W m-1C-1, 2.65 kJ kg-1 °C-1 and 1138.6 kg m-3, respectively.
The results of the thermophysical properties of the almonds are in table 4. Consequently, the results of the thermophysical properties show that the almond has characteristics for the easy removal of water in roasting, drying, and heating processes in unit operations, for use in flour or candied products, where the most used mathematical model to know the thermophysical properties is the one developed by Choi and Okos (1986), based on the temperature range from -40 to 150ºC and the composition of the food: moisture, protein, fat, fiber, carbohydrates and ash, which were compared with Deproter to determine the thermophysical properties.
Table 4. Thermophysical properties of almonds for industrial uses.
| Property | Thermal conductivity | Specific heat | Diffusivity | Density |
|---|---|---|---|---|
| Units | K (W m-1 C-1) | Cp (KJ kg-1 °C-1) | α (m2s-1) | ρ (kg m-3) |
| Dried almond | 0.32±01 | 2.65±03 | 1.13·10-7±002 | 1138.6±004 |
Compared to other products that are used in heat processing operations, such as coffee, with a specific heat between 1.442 kJ kg-1 K-1 to 3.298 kJ kg-1 K-1, conductivity between 0.117 Wm-1 K- 1 to 0.204 W m-1 K-1 and average diffusivity of 1.671·10-7 m2 s-1 (Casanova et al., 2013), the almond has adequate characteristics for heating and use in transformation operations. Compared to some varieties of cocoa such as Theobroma grandiflorum that has 0.51 Wm-1C-1, specific heat of 2.86 KJ kg-1K-1, and diffusivity of 9.94·10-10 to 6.29·10-10 m² s-1 (Cunha et al., 2021), the almond is allows a good distribution of heat in its structure. Taking into account the number reported by Luikov (1966) and referenced by Ferreira and Costa (2009) for the thermophysical properties of the almond, it was confirmed that the internal transfer of matter dominates the simultaneous transfer of heat and matter. During the confit process, temperatures of 80-90°C are used to dry the syrup and provide color to form the protective shell of the almond. The results for the thermophical properties will help control the indicated temperatures in order to not alter the nutritional composition of the final product. For candied almonds, the statistical analysis showed that there were statistically significant differences between the treatments (P<0.05) with respect to the appearance and taste of candied almonds.
Concentration levels of cyanogen and hydrocyanic acid found in the different samples
A reliable and simple method of determination was applied, which also allowed to separately quantify the cyanogenic compounds that were in the almond seeds, using high-performance liquid chromatography (HPLC) because it quantifies glycosides separately, and microdiffusion was chosen as the reference method. For the question of whether to add β-glucosidase or not, there is controversy; some authors (Armstrong et al., 1908) claim that the almond contains enough of the enzyme Emulsin (EC 3.2.1.21) to achieve hydrolysis without the need for the addition of external enzymes; others suggest adding β-glucosidase in microdiffusion assays. Table 5 shows the mean concentrations of the cyanogenic compounds in the analyzed almonds, using HPLC. Figure 1 shows a chromatogram of a standard mixture of amygdalin and prunasin obtained using a Symmetry C18 column; depending on the concentration levels of these glycosides, sweet, slightly bitter or bitter almonds were determined, with a direct correlation between concentration and degree of cyanides. Given the results using HPLC to determine the cyanogenic compound amygdalin, the sweet ‘Atocha’ presented a concentration of 7.65 mg/100 g, while ‘Ferraduel’ (sweet) presented a concentration of 23.37 mg/100 g, almost the same amount as the slightly bitter ‘Garrigues’ with 23.37 mg/100 g.
Table 5. Mean values (mg/100 g sample) of amygdalin content obtained for each variety studied in each harvest year.
| Sample | Variety | Taste | Year 1 | Year 2 | Total | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| 1 | ‘Desmayo L.’ | Sweet | 8.11 d | 0.09 | 6.86 d | 0.10 | 7.49 d | 0.88 |
| 2 | ‘Del Cid’ | Sweet | - | 2.15 b | 0.04 | 2.15 b | 0.04 | |
| 3 | ‘Atocha’ | Sweet | 7.28 d | 0.04 | 8.02 d | 0.13 | 7.65 d | 0.06 |
| 4 | ‘Ferragnès’ | Sweet | 5.16 c | 0.10 | 5.5 c | 0.16 | 5.33 c | 0.04 |
| 5 | ‘Peraleja’ | Sweet | 1.76 a | 0.20 | 2.56 a | 0.37 | 2.16 a | 0.12 |
| 6 | ‘Primorskii’ | Sweet | nd | - | nd | - | nd | - |
| 7 | ‘Marcona’ | Sweet | 1.87 a | 0.10 | 1.87 a | 0.27 | 1.87 a | 0.12 |
| 8 | ‘Ramillete’ | Sweet | nd | - | nd | - | nd | - |
| 9 | ‘Ferraduel’ | Sweet | 21.96 h | 0.53 | 23.36 h | 0.53 | 22.66 h | 0.00 |
| 10 | ‘Achaak’ | Sweet | 10.22 e | 0.03 | 11.25 e | 0.37 | 10.74 e | 0.24 |
| 11 | ‘Planeta’ | Sweet | 3.71 c | 0.16 | 4.65 c | 0.09 | 4.18 c | 0.05 |
| 12 | ‘Bonita’ | Sweet | nd | - | nd | - | nd | - |
| 13 | ‘Colorada’ | Sweet | 2.41 b | 0.22 | 2.72 b | 0.07 | 2.56 b | 0.10 |
| 14 | ‘Carretas’ | Sweet | 2.49 b | 0.32 | 2.65 b | 0.19 | 2.57 b | 0.09 |
| 15 | ‘La Mona’ | Sweet | 4.63 c | 0.31 | 6.10 c | 0.10 | 5.37 c | 0.15 |
| 16 | ‘Tioga’ | Sweet | 0.34 a | 0.04 | 0.52 a | 0.02 | 0.43 a | 0.01 |
| 17 | ‘Titan’ | Sweet | 0.43 a | 0.04 | 0.62 a | 0.02 | 0.53 a | 0.01 |
| 18 | ‘CEBAS’ | Sweet | nd | - | nd | -. | nd | - |
| 19 | ‘Pajarera’ | Sweet | - | - | 27.26 f | 1.14 | 27.26 f | 1.14 |
| 20 | ‘Rumbeta’ | Sweet | - | - | 5.39 c | 0.10 | 5.39 c | 0.10 |
| 21 | ‘Garrigues’ | Slightly bitter | 23.81 f | 0.54 | 22.93 f | 0.44 | 23.37 f | 0.07 |
| 22 | ‘Genco’ | Slightly bitter | 18.74 f | 0.09 | 17.34 f | 0.10 | 18.04 f | 0.01 |
| 23 | ‘Tuono’ | Slightly bitter | 25.05 f | 0.44 | 25.81 f | 0.27 | 25.43 f | 0.12 |
| 24 | ‘S3060’ | Bitter | 4915 i | 35.2 | 5036 i | 57.73 | 4976 i | 5.93 |
| 25 | ‘S3062’ | Bitter | 3870 h | 86.24 | 3784 h | 66.88 | 3827 h | 3.69 |
| 26 | ‘S3076’ | Bitter | 5894 g | 84.48 | 6028 g | 19.01 | 5961 g | 6.29 |
| 27 | ‘S3108’ | Bitter | 3799 h | 21.72 | 3810 h | 24.64 | 3805 h | 2.07 |
| 28 | ‘S3112’ | Bitter | 5206 g | 55.49 | 5011 g | 62.94 | 5109 g | 5.27 |
| 29 | ‘S3126’ | Bitter | 2439 f | 46.66 | 2360 f | 131.09 | 2400 f | 9.70 |
Means with different letters indicate significant difference according to the HSD Tukey test (P≤0.05). SD: Standard deviation.
The concentrations found in the studied almonds did not mean that they were toxic since the conversion of cyanogen to hydrocyanic acid was close to 12-14% (Arrázola et al., 2013). Many communications describe suicide attempts by ingesting cyanide compounds but generally do not state the doses. The average lethal dose by ingestion in humans is estimated at 200 mg of CNK or CNNa (Egekeze and Oehme, 1980; Ansell and Lewis, 1970). The sweet almond contains small amounts (~ 0.2 to 16 mg/100 g of almond) of amygdalin, while the bitter almond has a high level of this glucoside (2400 to 5970 mg/100 g), a precursor of hydrocyanic acid (Arrázola et al., 2015). Authors such as Briggs and Yuen (1978) have described how the effect of soaking on cyanogenic glycosides; for example, in apricot seeds, it decreases the total cyanide content by 13-52% after 24 h, 73-75% after 48 h, and 90% after 72 h (Tuncel et al., 1995); the endogenous β-glucosidase activity induces a significant degradation of amygdalin in apricot seeds that are ground and soaked at 20°C. That is, there are physical means by means of temperature and heat to control the final concentration of hydrocyanic acid in a product to be consumed either by people or animals. Table 6 shows the results from the analysis with HPLC and microdiffusion; these results were equivalent to the total cyanide contributed by the cyanogenic compounds, obtained from samples taken on day 83 to day 240, where the maximum concentration obtained for cyanide, with an average of 375.40 mg/100 g of cyanide. The importance of the processing temperatures must be considered in each use of the different components of sweet, slightly sweet, and bitter almonds for controlling hydrocyanic acid, given its volatility.
Table 6. Mean values of amygdalin and prunasin contents, total cyanide obtained with HPLC (amygdalin and prunasin equivalent) and total cyanide with microdiffusion, all expressed in mg of cyanide/100 g of sample.
| Sample | Julian day | HPLC | Microdiffusion | ||||
|---|---|---|---|---|---|---|---|
| Cyanide (amygdalin) | Cyanide (prunasin) | Total cyanide (amygdalin+prunasin) | Total cyanide | ||||
| Mean | Mean | Mean | SD | Mean | SD | ||
| ‘S3067’ | 83 | 2.10 | 17.30 | 19.40 | 0.14 | 8.20 | 1.13 |
| 118 | 15.20 | 32.45 | 47.65 | 1.70 | 43.48 | 2.05 | |
| 146 | 97.98 | 44.25 | 142.20 | 0.80 | 138.30 | 1.90 | |
| 180 | 322.65 | 38.50 | 361.20 | 2.70 | 352.50 | 2.03 | |
| 210 | 374.25 | nd | 374.30 | 2.30 | 372.00 | 4.90 | |
| 240 | 381.80 | nd | 381.80 | 4.00 | 375.40 | 5.30 | |
| ‘S3056’ | 83 | 0.77 | 7.43 | 8.20 | 0.14 | 10.40 | 1.98 |
| 118 | 5.20 | 14.70 | 19.90 | 3.88 | 22.20 | 4.20 | |
| 146 | 43.90 | 25.95 | 69.85 | 0.45 | 74.20 | 1.79 | |
| 180 | 151.20 | 20.05 | 171.25 | 8.20 | 170.55 | 1.00 | |
| 210 | 186.40 | nd | 186.40 | 5.52 | 186.50 | 2.80 | |
| 240 | 190.20 | nd | 190.20 | 2.44 | 189.38 | 8.30 | |
| ‘Genco’ | 83 | nd | 0.05 | 0.05 | nd | 0.05 | 0.01 |
| 118 | 0.04 | 0.08 | 0.11 | 0.02 | 0.14 | 0.04 | |
| 146 | 0.11 | 0.12 | 0.23 | 0.06 | 0.28 | 0.10 | |
| 180 | 0.75 | 0.10 | 0.85 | 0.02 | 0.81 | 0.07 | |
| 210 | 0.89 | nd | 0.89 | 0.10 | 0.91 | 0.09 | |
| 240 | 0.97 | nd | 0.97 | 0.06 | 0.95 | 0.03 | |
| ‘Marcona’ | 83 | 0.01 | nd | 0.01 | 0.01 | nd | - |
| 118 | 0.01 | nd | 0.01 | <0.01 | 0.01 | <0.01 | |
| 146 | 0.03 | nd | 0.03 | <0.00 | 0.03 | <0.01 | |
| 180 | 0.10 | nd | 0.10 | <0.01 | 0.10 | <0.01 | |
| 210 | 0.11 | nd | 0.11 | <0.01 | 0.11 | <0.01 | |
| 240 | 0.11 | nd | 0.11 | <0.01 | 0.10 | <0.01 | |
Source: Arrázola et al. (2013).
In other fruits of the same family, the cyanide level can cause serious acute problems that can lead to death, for example, raw or improperly processed apricots (Haque and Bradbury, 2002). Other studies have shown that apricot kernels contain a cyanide (CN) content of 1450 mg kg-1, approximately 0.5 mg g-1 (Mandenius et al., 1983). This value is similar to the toxic dose of cyanide (0.5 mg kg-1 body weight) indicated by WHO (2004).
Table 7 shows the mean cyanide values for the samples with high and low cyanide contents; they did not differ practically between the two methods. The standard deviation values were also very similar. On the other hand, it is worth highlighting that the correlation obtained between the data corresponding to the two methods was high and significant for both sets of samples, important for industrial uses of almonds as raw material for various derivatives. When making food from cyanogenic plants stored at room temperature (35±2°C), the cyanide content of food volatilizes because of the low evaporation temperature (26°C) described by Onabolu et al. (2002), with a 50-64% decrease in the cyanide content of cassava products (Gari) stored for 4 weeks at room temperature. The values obtained with the analyses for the bitter almonds (265.78-262.95 mg/100 g dry of sample) showed a great difference between the concentrations of the sweet and slightly bitter almonds but they can still be used as raw material or as a final product if they are subjected to treatments to guarantee volatilization as cyanhydric acid.
Table 7 Mean values of cyanide content (mg/100 g of dry sample) and standard deviations. Pearson's correlation coefficients and their significance.
| Valor | Sweet samples and slightly bitter samples | Bitter samples | ||
|---|---|---|---|---|
| HPLC | Microdiffusion colorimetry | HPLC | Microdiffusion gravimetric titration | |
| Average | 4.72 | 4.53 | 265.78 | 262.95 |
| Standard deviation | 6.54 | 6.27 | 74.85 | 71.5 |
| Correlation coefficient | 0.980 | 0.993 | ||
| Correlation significance | < 0.001 | < 0.001 | ||
CONCLUSIONS
The results showed that these varieties of almonds, especially the bitter fruits, did not present any danger of intoxication through direct consumption. The maximum concentration (375.40 mg/100 g) in the flour did not reach harmful levels that would produce immediate intoxication. However, constant consumption could be cumulative, and it would be necessary to evaluate the degree of affectation at the physiological level. On the other hand, during processing, especially when the compounds are subjected to temperatures higher than 26°C, hydrocyanic acid volatilizes. For the thermo-physical characteristics, it was determined that the internal transfer of matter dominates the simultaneous transfer of heat and matter, providing an adequate conduction of heat in transformation operations for almonds. Today, almonds are enjoying a trend of consumption in healthy diets, including candied almonds.