INTRODUCTION
Pineapple, Ananas comosus Merr. (Bromeliaceae), is native to southern Brazil and Paraguay where it was domesticated and then was taken northward through South and Central America to Mexico and the West Indies by the indigenous people prior to the arrival of Europeans (Morton, 1987). Currently, pineapple is produced in tropical and subtropical regions around the world. According to FAOSTAT (2021), based on the average production (in metric tons) between 2014-2019, the top 10 pineapple producers worldwide are Brazil (2,193,653 t), Thailand (2,139,920 t), Philippines (2,042,208 t), Costa Rica (1,773,360 t), India (1,373,663 t), Indonesia (1,135,412 t), Nigeria (1,126,262 t), mainland China (994,521 t), Mexico (660,171 t), and Colombia (506,365 t). Based on the figures of the National Agricultural Survey, in 2015, there were a total of 8,871 ha planted for the cultivation of pineapple in Colombia, of which the department of Valle del Cauca reported the highest production with 35.22%, followed by Quindio with 25.29%, Santander 11.68%, Cauca 10.82% and Casanare with 7.20%; the remaining 9.79% were produced by the other 12 departments with shares each below 2.49%, such as Antioquia, Bolivar, Boyaca, Cesar, Cordoba, Cundinamarca, Huila, Meta, Nariño, Risaralda, Sucre, and Tolima (DANE, 2016).
Various insect pests have been reported affecting pineapple crops in Colombia, including the weevils Metamasius dimidiatipennis (Jekel) and M. hebetatus (Gyllenhal) (Coleoptera: Curculionidae: Dryophthorinae), the pink pineapple mealybug Dysmicoccus brevipes (Cockerell, 1893) (Hemiptera: Pseudococcidae), the pineapple hairstreak Strymon megarus (Godart) (as Strymon basilides) (Lepidoptera: Lycaenidae), the sugarcane midget Elaphria nucicolora (Guenée, 1852) (Lepidoptera: Noctuidae), and the pineapple fly Melanoloma viatrix Hendel, 1911 (Diptera: Richardiidae) (Villalobos-Moreno et al., 2009; Mosquera, 2016). Of these, in the department of Valle del Cauca, M. dimidiatipennis, S. megarus and D. brevipes are considered the main insect pests of pineapple (Mesa-Cobo et al., 2014).
During a survey carried out in 2019 to determine the prevalence of mealybugs associated with two agroclimatic zones of pineapple production in the department of Valle del Cauca, Colombia, two natural enemies were collected which were identified as the encyrtid wasp Hambletonia pseudococcina Compere, 1936 (Hymenoptera: Encyrtidae) and the drosophilid fly Pseudiastata sp. (Diptera: Drosophilidae).
According to the Universal Chalcidoidea Database (Noyes, 2019), H. pseudococcina is widespread in the New World, recorded from the Antilles, Argentina, Bermuda, Brazil (Goias, Rondonia, Sao Paulo), Colombia, Costa Rica, Dominica, Dominican Republic, Ecuador, Jamaica, Mexico, Puerto Rico, Trinidad and Tobago, United States of America (California, Florida), and Venezuela. Outside the Americas, it is known from Africa (Ghana), the South Pacific region (Hawaii) and the Palaearctic region (Japan, Taiwan) (Noyes, 2019). Although not recorded in the Chalcidoidea database, H. pseudococcina has been recorded also in Cuba (Ceballos and Granada, 2004). Hambletonia pseudococcina has been recorded previously in Colombia from the departments of Caqueta reared from Pseudococcus sp. (likely Dysmicoccus) (Hemiptera: Pseudococcidae) on pineapple (Sharkov and Woolley, 1997), and Valle del Cauca, from Puto barberi (Cockerell, 1895) (Hemiptera: Putoidae)on coffee roots (Gil et al., 2016). This is the first report of H. pseudococcina from D. brevipes on pineapple in the department of Valle del Cauca.
The genus Pseudiastata Coquillett (Diptera: Drosophilidae) is New World in origin, known from the Mid-Atlantic region of the United States, down to Central and South America (Brake and Bächli, 2008). Pseudiastata includes six species, namely P. armata Wheeler, P. australis Blanchard, P. brasiliensis Costa Lima, P. nebulosa Coquillett, P. pseudococcivora Sabrosky, and P. vorax Sabrosky (Brake and Bächli, 2008). All known species of Pseudiastata, with the exception of P. nebulosa, the type species of the genus, and P. armata, have been recorded preying exclusively on pineapple mealybugs (Hardy, 1959). The insect hosts of P. armata and P. nebulosa are unknown. This is the first record of the genus in Colombia.
Here we document the first report of the parasitoid wasp H. pseudococcina and the predatory fly Pseudiastata sp., associated with the mealybug D. brevipes, in the department of Valle del Cauca and provide brief diagnoses for the recognition of these natural enemies.
MATERIALS AND METHODS
Collecting locality
Both the parasitoid wasp and the predatory fly were collected in association with pineapple mealybugs, which were found at the base of the crown of a pineapple fruit collected at Las Veraneras farm, municipality of Vijes, department of Valle del Cauca, Colombia (03°05′26″ N, 76°28′53″ W, 1,455 m a.s.l.). The infested fruit was found at the edge of a newly planted batch of pineapple crop within a pile of discarded plants from the previous crop.
Sampling and specimen preservation
The pineapple skin with the mealybug infestation and associated natural enemies was cut out from the fruit with a pocketknife, placed into a 200 mL plastic container and taken to the laboratory. Then the samples were transferred to a different plastic container with a dry paper towel on the bottom and sealed with a mesh lid to allow air flow. The drosophilid fly pupae were found near the mealybugs and were kept in the same plastic container with the parasitized mealybugs until they emerged. The parasitized mealybugs and fly pupae were observed daily, and the emerged parasitoids and flies were placed in vials with 75% ethanol (EtOH). A few mealybug mummies (= dead parasitized mealybugs that become brownish to reddish brown in color) were dissected in order to photograph the developing parasitoids. Some samples of the encyrtid parasitoids were sent to the second author (J.B.W.) for identification.
Mass rearing of Hambletonia pseudococcina
Several specimens of D. brevipes parasitized by H. pseudococcina were taken to the entomology laboratory at the Corporacion Colombiana de Investigación Agropecuaria - Agrosavia, Palmira Research Station, located in the municipality of Palmira, Valle del Cauca, Colombia, 03°31′17″ N, 76°18′25″ W, ca. 1,000 m a.s.l., to start a rearing colony on mealybugs of D. brevipes kept on pumpkin, Cucurbita moschata Duchesne (Cucurbitaceae). The insects were reared under laboratory conditions (25.9 ± 1.7°C, with 67.8 ± 8.1% RH, and natural light regimen, approximately 12:12 h [L:D]). The pumpkins were stored in a plastic container with a mesh lid, with a dry paper towel on the bottom. The reared parasitoids were used for observations and for providing voucher specimens.
Specimen preparation and identification
Encyrtid wasp
The emerged parasitoids from the sampled mealybugs were preserved in 75% ethanol (EtOH). Some alcohol-preserved specimens were critical-point-dried and mounted following the method described by Noyes (1982). Some specimens were point-mounted without previous treatment and others were mounted on slides in Hoyer’s mounting medium (distilled water 50 cm3, gum Arabic 30 cm3, chloral hydrate 200 cm3 and glycerin 20 cm3). The antennae were removed prior to the treatment of the specimens and used to determine the distribution pattern of the coloration. The specimens used for slide-mounting were cleared by heating them ca. 15 min in 10% w/v KOH prior to mounting. The specimens were identified to genus and species level by using the keys of Noyes (2000) and Sharkov and Woolley (1997). Morphological terminology used for the parasitoids follows that of Gibson (1997).
Drosophilid fly
The external morphology of the drosophilid flies kept in 75% ethanol was observed under a Nikon SMZ 745T stereomicroscope. The specimens were identified to the genus level using the diagnoses provide by Hardy (1959) and Sabrosky (1951). Only two adult females emerged, and because males are critical for the taxonomy of these flies, species level identification was not possible. Sabrosky (1951) studied the adults of four species, namely Pseudiastata nebulosa, P. pseudococcivora, P. vorax, and an undescribed species, which he listed as Pseudiastata sp., and concluded that they were all virtually indistinguishable based on superficial characters. He pointed out that the differences in the wing markings were not useful for the separation of species because they were all very similar, differing only in extent and intensity, and that the fundamental pattern of the above species was the same (Sabrosky, 1951). Sabrosky (1951) also stated out that species separation of the adult stage could be done by comparing the lateral and anterior aspects of the male terminalia and that females can be identified only by association with male specimens.
Mealybugs
The mealybug specimens were slide-mounted following the methods of Williams and Granara de Willink (1992). The specimens were identified to species level using the keys of Caballero et al. (2017), Pacheco da Silva et al. (2020), Tanaka and Kamitani (2021) and Williams and Granara de Willink (1992).
Collecting permit
Insect samples were collected under a global collecting permit “Permiso marco de recolección de especímenes de especies silvestres de la diversidad biológica con fines de investigación científica no comercial” [Permit framework for collecting of specimens of wild species of the biological diversity for non-commercial scientific research purposes], resolution No. 1466, expedited on December 3, 2014, by the Autoridad Nacional de Licencias Ambientales (ANLA) [Colombian National Authority Environmental Permits].
RESULTS AND DISCUSSION
Diagnoses
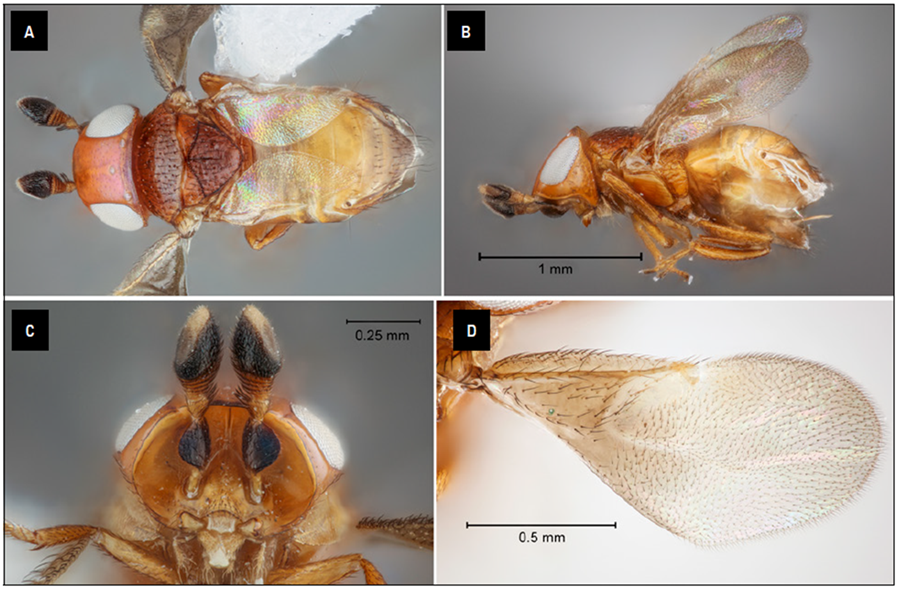
Hambletonia Compere is a distinctive genus in the subfamily Tetracneminae of Encyrtidae. It can be recognized by an orange or brown coloration of head and mesosoma (Fig. 1A), head strongly triangular in lateral aspect with a sharp occipital margin (Fig. 1B), and a sharply margined frontofacial ridge that continues ventrally as sharply margined antennal scrobes enclosing a concave facial area (Fig. 1C) (Noyes, 2000). Noyes (2000) diagnoses the subfamily Tetracneminae providing characteristics to distinguish them from the subfamily Encyrtinae. Sharkov and Woolley (1997) gave a detailed description of the genus Hambletonia and provided a key to world species.
Hambletonia pseudococcina Compere, 1936
Hambletonia pseudococcina can be diagnosed by the following combination of characters: i) anterior edge of frontovertex in dorsal view (occipital plane oriented vertically) distinctly convex (Fig. 1B); ii) frontovertex without prominence and punctures, or at most with scattered small inconspicuous punctures (Figs. 1A and B); iii) frontovertex more or less smooth between anterior ocellus and frontofacial ridge with only minute piliferous punctures; iv) frontovertex and eyes completely hairless, or with extremely minute hairs visible only at higher magnification when viewed and illuminated at certain angle, with at most a few very short hairs present behind posterior ocelli; v) setae on eyes at most about half as long as diameter of facet, vi) anterior and posterior ocelli situated in the same plane (Fig. 1A); vii) scape mostly dark brown; viii) pedicel with a tuft of elongate hairs on dorsal side (Fig. 1A), ix) tuft of setae on pedicel composed of very slightly broadened, almost scale-like setae (Fig. 1C); x) funicle 6-segmented; xi) clava entirely dark brown and less than 1.5X as long as broad with sensory area as long as dorsal surface of clava; xii)forewing length 2.0-2.5 times maximum width (Fig. 1D); xiii) basal cell of fore wing pilose, propodeal spiracle not reaching side of propodeum, its diameter less than diameter of mid basitarsus; longitudinal median basal groove on scutellum deep and about half as long as scutellum; and xiv) costal cell of forewing with several rows of setae dorsally (Fig. 1D) (Sharkov and Woolley, 1997).
Hambletonia pseudococcina is close to H. pilosifronsSharkov and Woolley (1997), H. roseniSharkov and Woolley (1997), and H. calvifronsSharkov and Woolley (1997). It differs from H. pilosifrons and H. roseni by the completely naked frontovertex and eyes, with at most minute hairs that can be present behind the posterior ocelli [ H. pilosifrons with frontovertex with translucent brownish semierect to erect hairs directed anteriorly and some posteriorly; and eyes with rather dense translucent hairs; and H. roseni with extremely minute hairs on the frontovertex and eyes]. It further differs from H. roseni by the pilose basal cell of the forewing [basal part of forewing almost hairless in H. roseni]. H. pseudococcina differs from H. calvifrons by the convex anterior edge of the frontovertex; the presence of several rows of setae on the dorsal surface of the costal cell of the forewing; scape dark colored and maximally broadened in the middle part; and eyes almost hairless [H. calvifrons can be diagnosed by the following character states: i) anterior edge of frontovertex almost straight; ii) costal cell of forewing with only a single row of setae; iii) scape slightly more elongated and light colored and maximally broadened in distal half;iv) scape entirely pale orange; v) clava with base pale orange and at least 1.8X as long as broad with sensory area less than 0.9X as long as dorsal margin of clava; vi) tuft of setae on pedicel composed of gradually tapering, bristle-like setae; vii) frontovertex and eyes with minute hairs;and viii) setae on eye nearly as long as diameter of facet].
In life, the dorsum of unparasitized pineapple mealybugs is pinkish, usually with well-developed lateral waxy filaments (Fig. 2A); ventral derm is yellowish pink, with yellowish limbs (Fig. 2B). Mealybug mummies parasitized by H. pseudococcina are almost spherical (Figure 2C-H), with brown to reddish-brown limbs and derm, and have noticeably less mealy wax on their surfaces (Figure 2D-I) compared to unparasitized individuals and often have shorter lateral wax filaments (Fig. 2C). One hundred mummies were studied. Most mummies (83%) had one parasitoid, however some mummies (17%) showed super-parasitism, with two parasitoids within when dissected; those mummies that contained two individuals within (Fig. 2F and I) were larger and much broader than those with only one individual within (Fig. 2E). Fig. 2I shows a mummy that had two parasitoid pupae within, of which one was extracted (Fig. 2J). The parasitoid pupa was of the exarate type, whitish, semitranslucent, with red eyes.
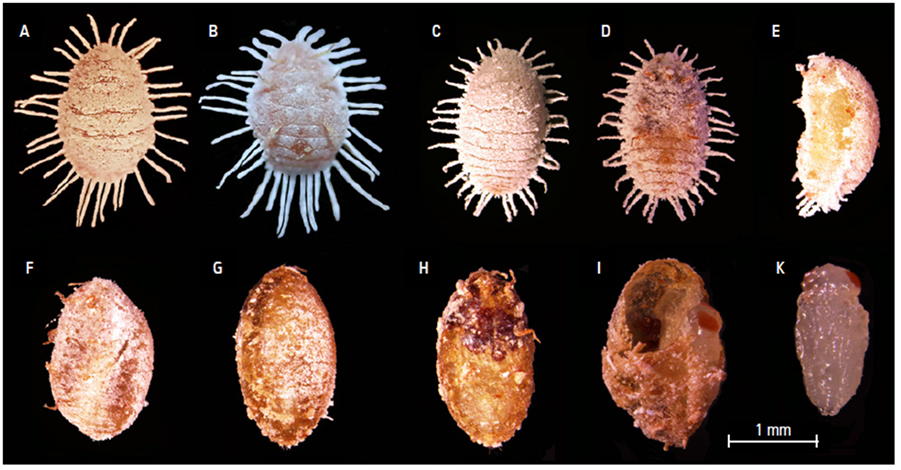
Photos: A and B by T. Kondo; C-I by Y. Campos-Patiño.
Figure 2. Unparasitized and parasitized specimens of D. brevipes by H. pseudococcina. A. Dorsal view of unparasitized mealybug. B. Ventral view of unparasitized mealybug. C. Dorsal view of parasitized mealybug. D. Ventral view of parasitized mealybug. E. Sideview of parasitized mealybug with single parasitoid. F. Sideview of mealybug with super-parasitism. G. Dorsal view of parasitized mealybug in advance stage of parasitoid development. H. Ventral view of parasitized mealybug in advance stage of parasitoid development. I. Parasitized mealybug with two parasitoid pupae, one of which was removed. J. Pupa of H. pseudococcina.
A small but odoriferous parasitoid
While processing the samples of H. pseudococcina, Y.C.P. noticed that the adult wasps produce a strong distinctive smell, which persisted in the Petri dishes even several weeks after they were removed. We asked eight persons (including Y.C.P. and T.K.) to describe the smell of H. pseudococcina, and their opinions were as follows: i) a sweet smell similar to coconut essence; ii) fermented wood; iii) cedar wood with a touch of honey; iv) a smell similar to that produced by dolichoderine ants; and v) wet cigarette butts, with a sweet honey scent. From all the above descriptions of the smell, the coconut essence smell was detected by five of them. This appears to be the first report of a distinctive smell associated with a member of the Encyrtidae. Further studies are needed to determine the composition of this odoriferous secretion and its purpose, e.g., whether it is a sex pheromone, a host marking pheromone, a deterrent for other parasitoids and predators, etc.
Pseudiastata Coquillett
The genus Pseudiastata can be diagnosed as follows. Head: eyes bare, large, occupying most of the area of head in profile; cheek linear; front with numerous short hairs; occiput distinctly concave; arista pubescent when seen under the microscope. Thorax: densely covered with hairs but not arranged in definite rows; scutellum bare but with bristles; mesopleuron and pteropleuron also bare; legs normal, short, preapical dorsal bristles present in at least some tibiae; wings each pictured (with markings), venation and color pattern distinct (Fig. 3), costa fractured in two places, with a strong bristle before each break, costa extending to fourth vein, discal and second basal cells merging, anal cell small. Chaetotaxy: 1 inner and 1 outer vertical, cruciate postvertical bristles short, cruciate ocellar bristles short, 3 pairs of fronto-orbital bristles (anterior pair convergent, the 2 posterior pairs reclinate), 1 humeral bristle, 1 presutural bristle, 1 + 1 notopleural bristle, 1 strong supraalar bristle, 2 postalar bristles, 2 posterior dorsocentral bristles, 1 prescutellar acrostichal bristle, 2 scutellar bristles, and 2 sternopleural bristles (Sabrosky, 1951).
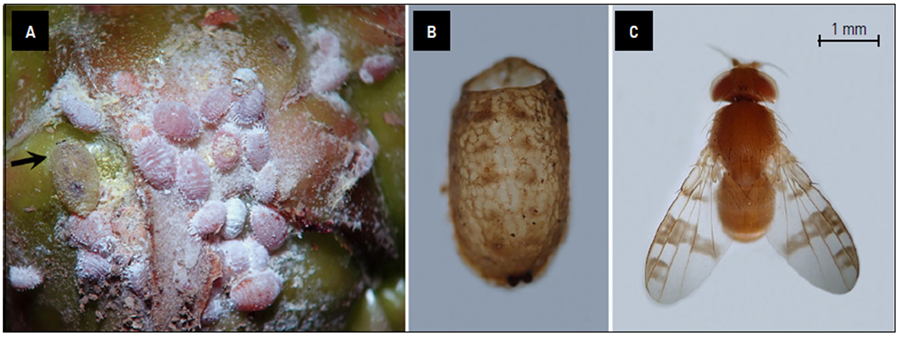
Photos: T. Kondo.
Figure 3 A. Aggregation of pineapple mealybugs near base of the fruit crown, with a pupa of Pseudiastata sp. on left side (see arrow). B. An empty pupa of Pseudiastata sp. C. Adult female Pseudiastata sp. Notice the characteristic color patterns on the wings.
The two Pseudiastata sp. specimens collected in the present study are each about 3 mm long. They match fairly well the generic diagnoses by Sabrosky (1951); however, further field studies are needed to collect male specimens of Pseudiastata in order to carry out a species level identification. A key to species of Pseudiastata can be found in Hardy (1959).
Material studied
Dysmicoccus brevipes (Cockerell) (Pseudococcidae), det. Takumasa Kondo, ex root crown of Ananas comosus L. cv. MD2 (Bromeliaceae), Colombia: Valle del Cauca, municipio Vijes, 03°45′ N, 76°28′ W, 1,455 m a.s.l., 11.iv.2019, coll. Takumasa Kondo, 2 slides: 10 adult females (CTNI: Catalogue No. 6693).
Hambletonia pseudococcina Compere, det. J.B. Woolley, Colombia: Valle del Cauca, Municipio de Vijes, 03°45′ N, 76°28′ W, 1,455 m a.s.l., 11.iv.2019, ex Dysmicoccus brevipes Cockerell found on fruit of pineapple, Ananas comosus L. cv. MD-2, coll. Yenifer Campos-Patiño, 12 female specimens, (TAMUIC: voucher specimen #758); 60 specimens in 75% alcohol (CTNI: Catalogue No. 6881).
Pseudiastata sp. (Diptera: Drosophilidae), det. Takumasa Kondo, Colombia: Valle del Cauca, Municipio de Vijes, 1,455 m a.s.l., 11.iv.2019, 03°45′ N, 76°28′ W, found next to an aggregation of Dysmicoccus brevipes Cockerell, on fruit of pineapple, Ananas comosus L. cv. MD-2, coll. Y. Campos-Patiño, 2 adult female specimens (CTNI: Catalogue No. 6682).
CONCLUSIONS
Two natural enemies were found in the department of Valle del Cauca, Colombia, in association with the pineapple mealybug, Dysmicoccus brevipes (Hemiptera: Pseudococcidae): an encyrtid parasitoid wasp, Hambletonia pseudococcina (Hymenoptera: Encyrtidae) and a drosophilid predatory fly, Pseudiastata sp. (Diptera: Drosophilidae). The genus Pseudiastata is for the first time recorded from Colombia and an odoriferous secretion is for the first time reported in a member of the family Encyrtidae. Further studies are needed to explore the rich biodiversity of natural enemies in Colombia which have the potential to be incorporated into biological control programs.