INTRODUCTION
Today more than ever, food security has become a relevant global concern, which has led to increased efforts to improve productive agricultural systems, in terms of quantity and quality (FAO, 2017). The challenge of agriculture in the world today is not only aimed at supplying the basic needs of the population, but also at conserving the environment and natural resources.
The productivity and dynamics of terrestrial ecosystems is limited to the availability and mobility of nutrients in the soil conditioned by air and water (Hou et al., 2012). For plants, the availability of nitrogen (N) is the main limiting factor in crop productivity, together with phosphorus (P), as both determine plant growth. Plants absorb nitrogen by the root as ammonium ion (NH4 +) or as nitrate ion (NO3 -) (Ceballos-Aguirre et al., 2022).
Nitrogen is a constituent of each of the amino acids, that is, it is present in each protein; it is also part of the chlorophyll molecule and nucleic acids (Okumoto and Pilot, 2011). Nitrogen stimulates the growth of stems and leaves. In addition, it stimulates the production of proteins in fruits and grains, and helps the plant to use other nutrients such as phosphorus and potassium (Jiaying et al., 2022). In a state of nitrogen deficiency in plants, it presents its first symptoms in mature leaves, its deficiency causes loss of turgor and color changes in the leaves, which first turn light green, then present chlorosis and finally die; root systems are reduced (Jiaying et al., 2022).
To increase the availability of these nutrients and improve crop productivity, chemical synthesis fertilizers are introduced into the soil; although their use is critical for food production, today it has become a costly practice that carries serious environmental consequences (Sumraj and Padhye, 2017). Such as the anthropogenic increase in greenhouse gas emissions and its influence on the planet, nitrous oxide (N2O) is a gas with a warming potential 298 times greater than CO2 (Sanjuán and Moreno, 2010). It is emitted by various economic sectors, including agriculture and livestock due to the use of nitrogen fertilizers (Garzón and Cárdenas, 2013). Likewise, in agricultural lands, nitrogen is mainly found as nitrate (NO3 -). Nitrates are not strongly retained in the soil, but rather have high mobility through the flow of water. Consequently, it is common for nitrates to move in depth with the drainage water, being able to reach the aquifers (Ceballos-Aguirre et al., 2022). Due to the Nitrogen cycle, the presence of nitrates and nitrites in the environment occurs naturally; however, human activities modify their concentrations and can make these compounds potentially dangerous to human and animal health (Okumoto and Pilot, 2011).
It is estimated that biological fixation contributes globally 180 million metric tons of ammonia per year and that the current contribution of anthropogenic nitrogen is comparable to the biological contribution (Beneduzi et al., 2013). Since its discovery, the importance of biological fixation for crop productivity and sustainability has been recognized (Rees et al., 2005). Microorganisms (solubilizers of phosphates and diazotrophs, among others) play the main role in many processes that involve the transformation of N and P, associated with effects of plant stimulation (phytostimulation and biofertilization), which in turn are influenced by the combination factors such as plant species, soil type and environmental conditions (Beneduzi et al., 2013). Many of these microorganisms establish pathogenic relationships with plants and can even favor their growth and resistance to biotic (against pathogens) and abiotic limitations (droughts, salinity, acidity, etc.). These are soil microorganisms, fungi, bacteria, archaea, etc. that are associated with the roots of plants in the functional domain known as rhizosphere, (Sanjuán and Moreno, 2010).
In accordance to their agronomic implications, ecological interactions between rhizobia and other soil bacteria have been of special interest in recent years. Further to a positive interaction between Rhizobium species and Diazotrophic soil bacteria such as Azotobacter and Azospririllum. According to Benintende et al. (2010) have reported increased nodulation and optimal growth of a wide variety of forages.
According to Pérez-Rodriguez et al (2020), tomato seed inoculation with A. brasilense (Az39) enhanced seedlings growth, which in turn could avoid the need of supplemental fertilization during greenhouse production. These same authors related that plant growth-promoting rhizobacteria inoculation allowed producing high-quality seedlings that likely suffered less transplant stress in the field, increasing yield and quality of tomatoes. Thus A. brasilense (Az39) can be used as bioinoculants implying significant economic savings for production, lowering groundwater and soil pollution and contributing to sustainable agriculture. Currently, one of the major challenges in tomato crop in the tropic is the correct application of bacterial inoculants in an open field for sustainable nutrient management (Bhardwaj et al., 2014). However, there are no reports concerning the effects of biofertilizer on commercial tomato hybrid. Therefore, it is necessary to perform field experiments with biofertilizer in order to increase crop yields while reducing the negative environmental impact of chemical fertilization. On the other hand, Chattopadhyay et al. (2022), B. japonicum (BRC 2485) was found to produce ABA, which plays a major role in triggering induced systemic resistance (ISR) in tomato plants. To the best of authors’ knowledge, this is the first report of a strain of B. japonicum with activity against bacterial wilt disease. The aimed to determine the effect of the interaction of Azospyrillum brasilense and Bradyrhizobium japonicumwith different levels of nitrogen on the yield and the economic feasibility of the tomato crop under semi-controlled conditions.
MATERIALS AND METHODS
This study was carried out at the Tesorito farm at the University of Caldas, located in the industrial park 11 kilometers from Magdalena road, western flank of the central Colombian mountain range, in the municipality of Manizales (Caldas), with an altitude of 2,340 m, average annual temperature of 17o C, relative humidity of 84.21 % and average annual precipitation 1,900 mm (Suárez et al., 2018).
An experimental design of divided plots was used, the largest plot being the dose of nitrogen fertilization (0, 50 and 100% of the nitrogen fertilization required by the crop) and the minor plot from the mixed liquid inoculant containing Bradyrhizobium japonicum (strain Semia 5079, 1 × 109 CFU mL-1) and Azospirillum brasilense (strains AbV5 and AbV6, 1 × 107 CFU mL-1) in the same formulation applied via seed treatment (0 cc ha-1; 100 cc ha-1; 200 cc ha-1 and 300 cc ha-1 of BFN) with four random internal blocks and five plants as experimental unit (Tab. 1).
Table 1. Interaction of nitrogen level and nitrogen-fixing bacteria (BFN) for each treatment.
| Percentage of nitrogen | Interaction % optimal nitrogen dose - dose of BFN (cc ha-1) | |||
|---|---|---|---|---|
| 0 BFN | 100 BFN | 200 BFN | 300 BFN | |
| 0 | 0-0 | 0-100 | 0-200 | 0-300 |
| 50 | 50-0 | 50-100 | 50-200 | 50-300 |
| 100 | 100-0 | 100-100 | 100-200 | 100-300 |
A commercial indeterminate chonto hybrid tomato was used. The sowing was carried out in trays of 128 locules with grade 3 sphagnum peat. The soil was prepared at a depth of 30 cm. When the seedlings had two to three true leaves, they were transplanted in the field under semi-controlled conditions and inoculated with a commercial mixture of nitrogen-fixing bacteria (BFN) based on Bradyrhizobium japonicum and Azospirillum brasilense; the levels of nitrogen fertilization were carried out according to the soil analysis of the test site and taking into account the nutritional requirements of the crop (Haifa, 2021) (Tab. 1). For 100% crop nutrition was carried out with the results of soil analysis and crop extraction. Two edaphic fertilizations were carried out during the crop cycle, one at the time of transplantation and the second 40 days after transplantation at a rate of 20 g/plant from the 10-20-20 source + 2 g/plant from mycorrhizae and the mixture of 2.4 g/plant (10-20-20) + 5.9 g/plant (KCl) + 11.8 g/plant (urea) + 5.9 g/plant (KNO3), respectively. For the 50% plots, we worked with 50% of the nitrogen from the nitrogenous sources mentioned above. And 0% without the addition of nitrogenous sources. The irrigation system was by dripping through irrigation tapes, with a distance between drippers of 30 cm and an average capacity per dripper of 30 cc min-1, where the water needs of the plants were supplied according to the phenological phases of the crop starting in the first weeks for each plant with 0.2 L day-1 and ending with 1.5 L day-1.Tutored system was individual with two axes and was carried out from the third week after the transplant. For all treatments, cultural work and phytosanitary control were carried out according to the methodology of Jaramillo et al. (2012).
The yield component variables were evaluated: number of clusters/plant, number of flowers/ cluster, number of fruits/plant, production/plant (g), and yield (t ha-1). Other variable was the analysis of production costs. Which it was recorded of the agronomic work were kept and the efficiency was evaluated, taking into account the time taken for each work. All the values in the calculation of the cost of production were quoted in Manizales, Caldas, in the fourth quarter of 2019. The characterization of production costs and economic analysis were carried out on records in a cost calculation sheet adopted from the model of the Colombia International Corporation in the Price Information System for the agricultural sector (Colombia DANE, 2021), collecting and recording the average price of the vegetable in the last 5 years at USD$0,38 k1. Infrastructure expenses, land preparation, seed purchase and sowing, crop maintenance, labor, supplies and equipment were accounted for, and gross income for each treatment and production costs for each system were calculated. To analyze the profitability of the crop, the following financial indicators were calculated: gross income, net income and production costs. The fixed and variable costs for each production system were incorporated, such as costs per hectare, Unit Production Margin (UPM), and cost benefit ratio (C/B R). The cost structure model was carried out according to Herrera et al. (2016).
The data obtained were evaluated by analysis of variance of two factors (nitrogen plot and dose of nitrogen-fixing bacteria-BFN), followed by Duncan's post hoc tests to allow comparisons of means by pairs (P<0.05). The significant differences between the two factors were determined separately and the interaction between both factors was established to determine whether or not it is significant, using the statistical program SAS, version 9.0 (SAS Inst. Inc. Cary, NC).
RESULTS AND DISCUSSION
For the variable number of clusters per plant, no significant differences were found (P<0.05) (Fig. 1 and Tab. 2) in any of the evaluated treatments and in their interaction. In the flowering of commercial and wild tomato, its genetic makeup is one of the relevant factors for the response of this variable (Restrepo et al., 2017); in the same way, the number of clusters found is within the ranges reported by Burbano and Vallejo (2017), who evaluated different lines of determined tomato with values of 15.80; 15.00 and 14.70 clusters/plant, considering these lines as promising for this variable.

Figure 1. Effect of nitrogen-fixing bacteria and % of optimal nitrogen dose on the number of clusters per plant in tomato crops.
Table 2. Analysis of variance for the yield components as effect of nitrogen-fixing bacteria and % of optimal nitrogen dose.
| Source | GL | N° clusters/ plant | N° flowers/ cluster | N° fruits/ plant | Yield (g/ plant) | Yield (t ha-1) |
|---|---|---|---|---|---|---|
| Nitrogen plot | 2 | 0.2866 NS | <.0001* | 0.0223* | <.0001** | <.0001** |
| Dose of BFN | 3 | 0.2372 NS | 0.2757 NS | 0.2851 NS | 0.0476* | 0.0476* |
| Plot*Dose of BFN | 6 | 0.8416 NS | 0.4847 NS | 0.072*** | 0.0096* | 0.0096* |
*Indicates significant statistical differences (P<0.05). **Indicates statistically significant differences (P<0.01). NS: indicates that there is no significance for the variable in the corresponding factor. *** For this case, statistically significant differences are reported (P<0.07).
For the variable number of flowers/cluster, no significant differences were found (P<0.05) for the treatments with BFN; however, significant differences were found (P<0.05) between the treatments with % optimal dose of nitrogen where the treatments with the highest number of flowers were the 100% N and 50% N treatments, with an average of 7 flowers/cluster, followed by the 0% N treatment, which has an average of 5 flowers/cluster (Fig. 2 and Tab. 2).

Figure 2. Effect of the % of the optimal dose of nitrogen on the number of flowers per cluster in the tomato crop. Different letters indicate statistically significant differences (P<0.05) with the Duncan-type mean test.
The variable of number of fruits/plant showed significant differences (P<0.05); the interaction of the treatment 0% N and 100 cc of BFN, obtained the highest number of fruits with 51 fruits/ plant (Fig. 3). The average of fruits found in this study agrees with reports by Moya (2005), between 37 and 89 fruits/plant, in cultivars with indeterminate growth, such as the one evaluated in this study. Although the 0% N treatment had a lower number of flowers, it had a better fruit set percentage (data not shown); therefore, a plant balanced between these two variables projects a good production, because the number of flowers/cluster, the number of fruits/plant and the average weight of the fruits are components of the tomato yield (Restrepo et al., 2017), which impacts the final yield of the crop. Similarly, Ceballos and Vallejo (2012) found that 80% of the 30 genotypes evaluated had a direct relationship between the number of flowers and fruits per cluster. The foregoing indicates that it is necessary to project a good number of flowers/cluster to promote the best fruit/cluster ratio and adequate filling for the sake of optimal crop performance, which goes hand in hand with adequate levels of nutrition.
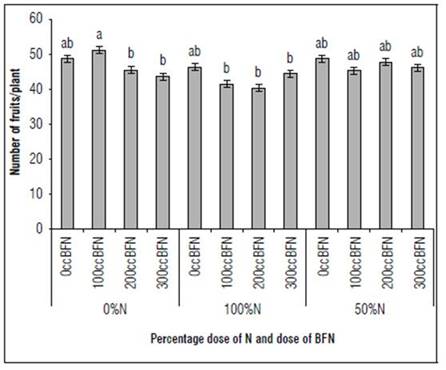
Figure 3. Effect of nitrogen-fixing bacteria and % of the optimal nitrogen dose on the number of fruits per plant in tomato cultivation. Different letters indicate statistically significant differences (P<0.07) with the Duncan-type mean test.
For the variable production per plant (Fig. 4), significant differences (P<0.05) were found in the interaction 0% N-100ccBFN that showed the highest production with 6,030 g/plant, followed by the interaction 50% N, with 200ccBFN for a production of 5,829 g/plant and 50% N with 100ccBFN reaching a production of 5,773 g/plant, showing that the tomato crop had a positive effect on its production in response to the application of BFN both in the absence and in the presence of nitrogen fertilization; this production is above the values found by Baena et al. (2003), who reported yield values in “chonto” tomato, which ranged between 3,328 and 5,904 g/plant.

Figure 4. Production per plant in tomato cultivation in response to the interaction of % optimal dose of nitrogen and dose of nitrogen-fixing bacteria (Azospyrillum brasilense and Bradyrhizobium japonicum). Different letters indicate statistically significant differences (P<0.05) with the Duncan-type mean test.
In relation to what was found in production per plant, the number of fruits per plant and the yield (t ha-1), a better performance is highlighted in the 0% N-100ccBFN interaction with a yield of 125 t ha-1 followed by the interactions 50% N with 0 cc ha-1 BFN, 100 cc ha-1 BFN, 200 cc ha-1 BFN with average yields of 120 t ha-1 (Fig. 5). These results demonstrate a great benefit in the application of BFN in crops such as tomato due to being able to reduce the application of nitrogen fertilization by 50% obtaining good yields and reducing the environmental impact that nitrogen fertilizers cause, on the other hand, the inoculation of BFN in plants leads to a significant increase in the root system, in addition to inducing resistance to pathogens and providing the necessary nitrogen, it also inhibits the proliferation of weeds and produces hormones that stimulate plant growth, which allows a more economical and healthy development of crops (Parra and Cuevas, 2001).
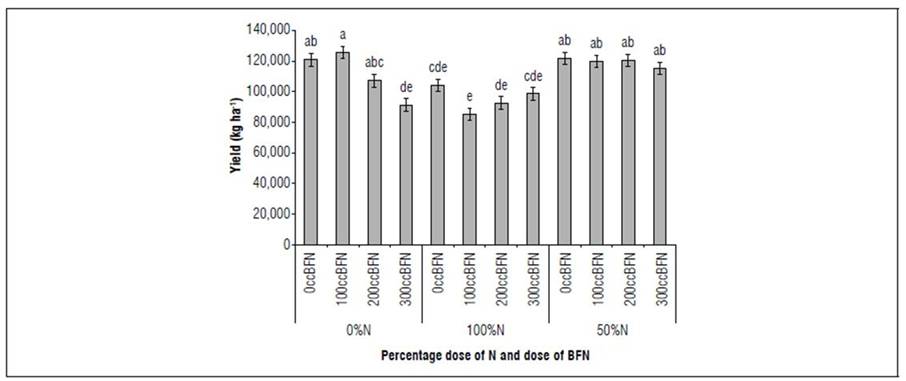
Figure 5. Yield in the tomato crop in response to the interaction % of the optimal dose of N and the doses of nitrogen-fixing bacteria (Azospyrillum brasilense and Bradyrhizobium japonicum). Different letters indicate statistically significant differences (P<0.05) with the Duncan-type mean test.
The behavior of the treatments with the applications of the BFN tends to increase the production of the crop, an effect already reported by Fornasero and Toniutti (2015), who affirms that the crop is nourished by the nitrogen provided by the BFN and from the nitrogen available in the soil, the BFN can contribute between 25 and 90% of the nitrogen necessary for the development of the crop.
The prevalent nutrients found in the development of the tomato plant and fruit are potassium, followed by nitrogen and calcium (Haifa, 2021), elements that in this work presented the interactions with the best performance, one of the properties that has been discovered in the inoculation with bacteria with the ability to fix nitrogen and solubilize phosphorus that have the ability to increase the availability of these two nutrients and also Fe and Zn, playing a fundamental role in reducing the dose of fertilizer needed from these elements (Singh et al., 2011).
It should be noted that nodules such as roots are continuously renewed and, as the nodules detach from the roots and decompose, they contribute their nutritional content to the soil, for which the BFN can have a great importance in the mineral nutrition of plants and, as a consequence, a positive effect in performance, as found in the present study.
Economic analysis
The economic analysis (Tab. 3) showed a better cost/benefit ratio for the 0% N-100ccBFN interaction with C/B 1.80, which is equivalent to a net income of USD$ 31,514.34. Although this interaction turned out to be the best economically, it is worth noting that for integral sustainability of the productive system it is not the most indicated, since this treatment does not have the application of a chemical nitrogen source that helps to sustain the nitrogen level of the soil (treatment with 0% nitrogen). On the contrary, when implementing this interaction, the reserve of soil organic matter would be depleted, therefore, interactions such as 50% N-100ccBFN and 50% -200ccBFN are an excellent economic option due to their C/B ratio of 1.64 and 1.65 respectively and for the economic and environmental sustainability that does not compromise the future fertility of the soil.
Table 3 Economic feasibility of tomato cultivation under semi-controlled conditions under the effect of nitrogen-fixing bacteria at different nitrogen levels (0-50-100 %).
| Nitrogen level | Treatment | Yield | Gross income (USD$) | Total cost (USD$) | UPM (USD$) | Net income (USD$) | C/B R |
|---|---|---|---|---|---|---|---|
| (t ha-1) | |||||||
| 100% Nitrogen / dose / plant | 0BFN | 104.0 | 39,411.19 | 17,820.06 | 0.17 | 22,633.25 | 1.27 |
| 100BFN | 85.0 | 32,193.97 | 17,920.25 | 0.21 | 14,962.65 | 0.83 | |
| 200BFN | 92.5 | 35,054.89 | 17,922.78 | 0.19 | 17,959.00 | 1.00 | |
| 300BFN | 98.5 | 37,327.37 | 17,925.32 | 0.18 | 20,338.50 | 1.13 | |
| 50% Nitrogen / dose / plant | 0BFN | 121.5 | 46,022.61 | 17,629.07 | 0.15 | 29,763.99 | 1.69 |
| 100BFN | 119.8 | 45,390.36 | 17,729.26 | 0.15 | 28,996.19 | 1.64 | |
| 200BFN | 120.3 | 45,567.57 | 17,731.79 | 0.15 | 29,179.29 | 1.65 | |
| 300BFN | 115.3 | 43,677.52 | 17,734.33 | 0.15 | 27,195.36 | 1.53 | |
| 0% Nitrogen / dose / plant | 0BFN | 120.7 | 45,730.17 | 17,418.04 | 0.14 | 29,678.64 | 1.70 |
| 100BFN | 125.6 | 47,581.54 | 17,518.23 | 0.14 | 31,514.34 | 1.80 | |
| 200BFN | 107.1 | 40,566.77 | 17,520.76 | 0.16 | 24,158.34 | 1.38 | |
| 300BFN | 91.3 | 34,595.04 | 17,523.30 | 0.19 | 17,895.72 | 1.02 |
UPM: unit production margin; B/C R: benefit/cost ratio
CONCLUSION
The interactions of Azospyrillum brasilense and Bradyrhizobium japonicumwith different levels of nitrogen demonstrated a synergistic action on the yield and economic feasibility of the corn crop, as is the case of the treatments 0% N-100ccBFN, 50% N-200CCBFN and 50% N-100ccBFN in all the yield variables, of which the most sustainable treatment (yield, economic and environmental) was 50% N-200ccBFN since it does not compromise soil fertility. The results found allow recommending the application of BFN in combination with adequate levels of nitrogen according to the cultivated species.















