INTRODUCTION
The forage cactus (Opuntia sp. or Nopalea sp.) is currently the main xerophyte plant cultivated in Brazil, where it is used as animal feed during the dry season in the northeast region of the country. Its high water-use efficiency, associated with its succulence, makes it an excellent energy and water source for many herd species (Almeida et al., 2019).
There are numerous types and cultivars of this cactus throughout the world (Almanza-Merchán and Fischer, 2012); however, in Brazil, there are only a few genotypes that are resistant to the pest Cochineal-carmine (Dactylopius opuntiae) and suitable for cultivation. Among the available genotypes, the forage cactus Mexican Elephant Ear [Opuntia stricta (Haw)] is notable in terms of rusticity, precocity and low soil fertility requirements. These characteristics can facilitate a high yield rate in this genotype, as compared to others (Araújo et al., 2019).
In contrast to other forage crops, the forage cactus is driven by the Crassulacean Acid Metabolism (CAM). The main advantage of plants with this mechanism is the time separation for (daytime) photosynthetic activity and (nighttime) stomata opening, when the release and uptake of CO2 take place. This adaptation allows the forage cactus to uptake and accumulate carbon during the period when less water loss occurs through transpiration activity (Black and Osmond, 2003).
The dynamics between forage cactus physiology and anatomy provides significant yield rates under both irrigated and non-irrigated cropping conditions. This is mostly possible because the CAM mechanism allows plants to save water and is the only photosynthetic pathway that ensures adaptation to periods of water shortage (Pimentel 2004; Silva et al., 2015). Rocha et al. (2017) worked under irrigated cropping conditions and obtained 556 Mg ha year-1 of forage cactus Mexican Elephant Ear with a density of 50,000 plants/ha. On the other hand, Souza et al. (2018) evaluated different genotypes under non-irrigated cropping conditions, with a density of 25,000 plants/ha, and reached a yield rate of 223.5 Mg ha year-1 in fresh mass.
Therefore, studies on gas exchange in plants with the CAM mechanism are essential to quantify stomata dynamics, i.e. the mechanism responsible for capturing and accumulating CO2 in plants. This is important for understanding photosynthetic, growth and water flux processes in terms of climate changes that can be triggered by seasonal variations.
Despite advances and technological innovations for agriculture management in Brazil, studies on forage cactus dynamics are lacking in the literature. The aim of this research was to assess the uptake of atmospheric CO2 and water-use efficiency in Opuntia stricta (Haw) during different periods of the year and times of the day.
MATERIALS AND METHODS
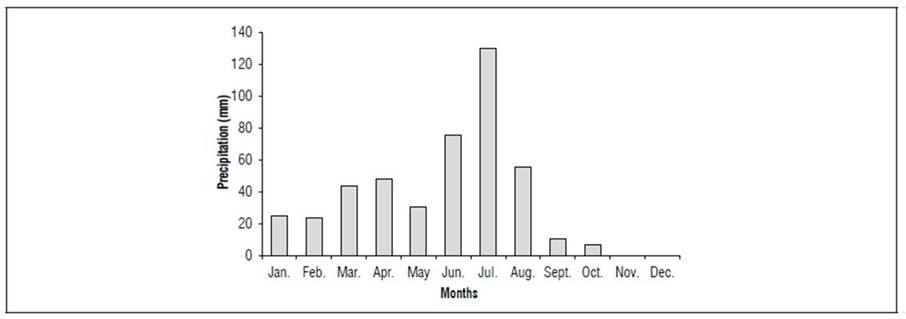
This study was conducted at the Ignácio Salcedo Experimental Station of the Instituto Nacional do Semiárdio - INSA, in the city of Campina Grande, State of Paraiba (more specifically, in the mesoregion of the Agreste paraibano); the coordinates are 07º13'50" S and 35º52'52" W, with an altitude of 551 m. The weather is classified as Aw’ type according to the Köppen climate classification, i.e. it is considered tropical with a water deficit during most of the year (Alvares et al., 2013). The data for rainfall numbers in 2019 are in figure 1. The total precipitation was 452.7 mm.
This study was carried out in a cropping area with a density of 20,000 plants/ha at 3 years after planting. The area has a variety of forage cactus Mexican Elephant Ears [Opuntia stricta (Haw)], which are resistant to Cochineal-carmine pest. The soil characteristics are in table 1.
Table 1. Soil chemical characteristics at 0-20 cm depth.
| pH | P | K+ | Na+ | H++Al+3 | Al+3 | Ca+2 | Mg+2 | V% | CTC | MO |
|---|---|---|---|---|---|---|---|---|---|---|
| (H2O, 1:2.5) | (mg dm-3) | (cmolc dm-3) | g kg-1 | |||||||
| 5.4 | 3.30 | 98.09 | 0.11 | 3.37 | 0.20 | 3.34 | 0.32 | 54.4 | 7.39 | 6.46 |
P, K and Na: Mehlich extractor 1; H + Al: Calcium acetate extractor 0.5 M, pH 7.0; H+Al: Calcium acetate extractor 0.5 M, pH 7.0; Al, Ca and Mg: KCl extractor 1 M; MO: Organic matter, Walkley-Black.
The treatments were distributed in a 24×2 factorial arrangement with 5 replications, corresponding to the evaluation of gas exchange fluxes for 24 h in the rainy (June) and dry (December) seasons. Each replicate was represented by a plant.
The following parameters were measured at two cladodes per plant: stomatal conductance (gs) (mol m-2 s-1), net CO2 uptake rate (A) (µmol m-2 s-1), transpiration rate (E) (mmol of H2O m-2 s-1) and internal CO2 concentration (Ci) (µmol of CO2 mol-1). With these data, it was possible to determinate the instantaneous (iWUE) and intrinsic (WUE) water-use efficiency, defined by the relationships between the net photosynthetic and transpiration rates (A/E), and the net photosynthetic rate and stomatal conductance (A/gs), respectively.
A portable infrared gas analyzer (IRGA) - model LCpro+ from BioScientific Ltd - was used to take the measurements. The procedure for the measurements using the IRGA consisted of determining the relative humidity, airflow rate and atmospheric CO2 concentration with a 6.25 cm2 leaf chamber. The photosynthetically active radiation, ambient and cladode surface temperature in both periods of the year are in figure 2.
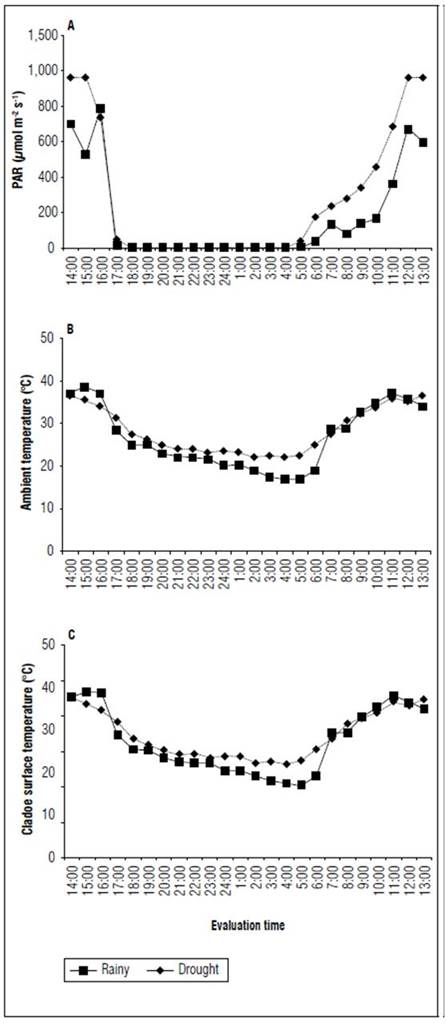
Figure 2. A. Photosynthetically active radiation (PAR), B. ambient and C. cladodes surface temperatures (°C) of the forage cactus Mexican Elephant Ear [Opuntia stricta (Haw)] at different times in the rainy and dry seasons.
The registered values were submitted to an analysis of variance applying the F-test. Once a significant difference was observed, the Tukey’s test was applied at a probability level of 5%. The data were processed using the statistical software SAS - Statistical Analysis System® (Cody, 2015).
RESULTS AND DISCUSSION
The forage cactus Mexican Elephant Ear presented a higher stomatal conductance (gs) at 1:00 in both evaluated seasons. The highest absolute values for the rainy and dry seasons were 0.602 and 0.198 mol m2 s-1, respectively (Fig. 3). In addition to the larger stomatal opening, about 204% larger in the rainy season, they also stayed opened for a longer time, opening at 15:00 (0.22 mol m² s-1) and closing at 8:00. (0.02 mol m² s-1). Throughout the dry season, the stomatal opening and closure occurred at 17:00 (0.01 mol m² s-1) and 6:00 (0.13 mol m² s-1), respectively.
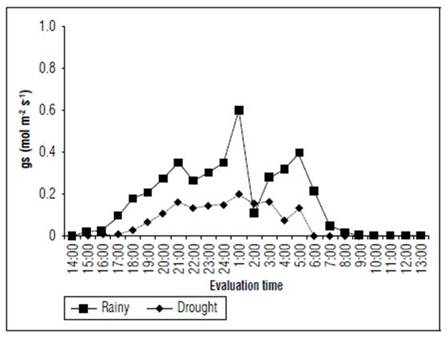
Figure 3. Stomatal conductance (gs) in the forage cactus Mexican Elephant Ear [Opuntia stricta (Haw)] at different evaluation times during the rainy and dry seasons.
The forage cactus is characterized as one of the main biological elements that are compatible with the ecology of the semiarid region of Brazil, which maintain local animal activities mostly through year-round permanence. This trait results from high water-use efficiency that is associated with pronounced CO2 uptake rates at night. In contrast to other native cactus, this one has fast growth, which is desirable for cultivation.
The highest stomatal conductance was probably induced by elevated edaphic and environmental moisture. Moreover, the low atmospheric and cladode surface temperatures and the reduction of the photosynthetically active radiation (Fig. 2) might have positively contributed to these results.
Stomatal conductance (gs) is a sensitive indicator, especially for changes in water content of the edaphic environment since the mechanism for closing stomata is probably a preponderant limiting factor to photosynthesis (Taiz et al., 2017). Because of the stress promoted by water shortages, ions and water transport systems take control with changes in the turgor pressure and stimulate stomata closure through membranes (Osakabe et al., 2014). It may be possible that this same behavior has been observed for forage cactus in the dry season. When the parenchyma water reservoir is depleted, and the efficiency of stomata in the chlorenchyma is reduced, the stomata start opening later and closing earlier (Taiz et al., 2017).
Flexas et al. (2014) noted that a water deficit was severe when the stomata conductance values were lower than 0.1 mol m-2 s-1, indicating that, for a dry season, the forage cactus is able to maintain stomata conductance even though their activity is low. The excellent adaptability of this xerophyte plant, which makes it the most cultivated one in Brazil, is associated with the Crassulacean Acid Metabolism (CAM), vapor pressure deficit and region characteristics, positively influencing the stomatal dynamics of forage cactus crops (Cajazeira et al., 2018; Almeida et al., 2019).
Pimentel (2004) confirmed that CAM plants can go long periods (from 100 to 200 d) without opening their stomata during the morning, consequently saving water. However, they will have a low accumulation of dry matter. Moreover, these plants have a stomatal frequency (around 2,500 stomata/cm2) eight times lower than C3 plants (approximately 20,000 stomata/cm2).
The CO2 uptake rate in the forage cactus between 18:00 and 8:00 throughout the rainy season was significantly higher than in the dry season. This behaviour was maintained during the rest of the day (Fig. 4). In the rainy season, the uptake of CO2 peaked at 24:00, 1:00 and 2:00, when the plants reached average values of 8.52, 8.58 and 9.51 μmol CO2 m-2 s-1, respectively, which, represented an increase of 647.3, 425.7 and 780.5%, in comparison to the values obtained (1.14, 1.67 and 1.08 μmol CO2 m-2 s-1) at the same evaluation times during the dry season. These results showed that, regardless of the season, gas exchange analyses must be accomplished within this range of time to attain a higher plant efficiency and good data reliability.
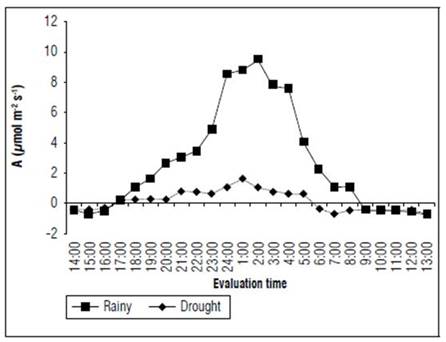
Figure 4. Uptake of atmospheric CO2 (A) in the forage cactus Mexican Elephant Ear [Opuntia stricta (Haw.)] at different evaluation times during the rainy and dry seasons.
Nobel (2009) explained that CAM plants have a maximum photosynthetic rate of 7.6 µmol CO2 m-2 s-1, whereas the normal rate is 2.5 µmol CO2 m-2 s-1. According to this study, some facultative CAM plants may reach high productivity levels by accumulating dry matter, as in the case of forage cactus, especially during periods with significant water availability.
The CO2 uptake at 17:00 and 8:00 indicated that, during the rainy season, the forage cactus Mexican Elephant Ear was a facultative CAM plant (Fig. 4) by increasing the uptake of carbon for a longer time (15 h d-1). This can positively influence phytomass increases in plants. Nevertheless, in the dry season, the plants not only decreased their capacities in absorbing carbon, also using less time for this activity (12 h d-1).
Winter and Holtum (2014) and Davis et al. (2019) reported that facultative CAM plants can undergo a certain physiological flexibility in relation to exclusive CAM plants. They verified that, when there are suitable water conditions, this is followed by the opening of stomata during the morning, which enables significant CO2 uptake rates with less energy depletion.
Despite the absence of moisture in the soil during the dry season, the forage cactus was able to maintain its physiological activities via a succulent xerophyte mechanism, even at a low intensity. To activate this mechanism, the plants suffer a gradual wilt process throughout the year. This reduction of water in the parenchyma reservoir at extreme levels may lead to substantial losses of stomatal activity in the chlorenchyma, as well as for the uptake of carbon (Souza et al., 2020).
Following this trend, the transpiration rates were more intense during the rainy season, reaching a minimum transpiration rate at 8:00 (-1.47 mmol m² s-1) and a maximum rate at 17:00 (1.61 mmol m² s-1). In the dry season, the values varied between -2.01 and 0.76 mmol m² s-1 from 14:00 to 1.00, respectively (Fig. 5). These values indicated that this cactus has different transpiration dynamics during the year. Despite the diverse climate changes, which took place during the evaluated periods, the transpiration rate was maintained at low levels because of stomatal opening. This fact was reasonably apparent at night, the period of the day where water loss was minimized through this pathway.
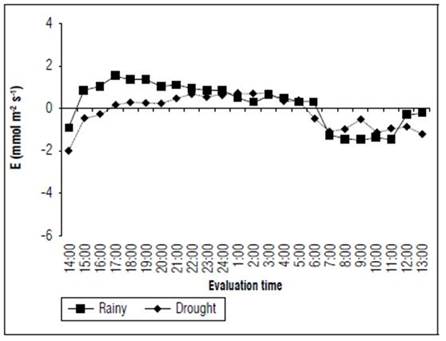
Figure 5 Transpiration rate (E) of the forage cactus Mexican Elephant Ear [Opuntia stricta (Haw)] at different evaluation times during the rainy and dry seasons.
For buffel grass (Cenchrus ciliares), a xerophyte grass driven by the C4 metabolism, Maranhão et al. (2019) observed a maximum transpiration rate of 4.84 mmol m² s-1 when irrigating at 113% of the evapotranspiration. Once the water blade thickness was reduced to 30%, the transpiration rate did not surpass 1.9 mmol m² s-1. For cowpeas (Vigna unguiculata (L.) Walp.), a leguminous plant with C3 metabolism, cultivated under non-irrigated and different edaphic conditions, Fernandes et al. (2015) identified a transpiration rate between 11 and 12 mmol m² s-1. When these plants are compared to CAM plants, such as the forage cactus, it was verified that a decrease in water loss occurred in this cactus even under favourable environmental conditions, such as the ones found during the rainy season. This was mainly due to the low vapour pressure deficit during the night, associated with the ability of this cactus to acquire water from the transpiration stream and dewdrops. Moreover, there is water storage in vacuoles (Souza et al., 2020). Under such conditions, the intracellular cavities are saturated with water vapors (a relative humidity of 100%), and the atmosphere generally has low relative humidity levels, as well as low water vapor contents.
The internal CO2 concentration was considerably higher during the dry season. Peaks of 388.2 μmol mol-1 at 19:00 and 1.539.6 μmol mol-1 at 24:00 were verified. In the rainy season, the average values varied between 303.8 μmol mol-1 at 14:00 and 722.6 μmol mol-1 at 10:00 (Fig. 6). From one period to another, the highest discrepancy occurred at noon, when the Ci of the plants during the dry season was 130.3% higher than in the rainy season. Higher Ci values were observed for the cladodes in the dry season, indicating that the CO2 was not utilized by the plants.
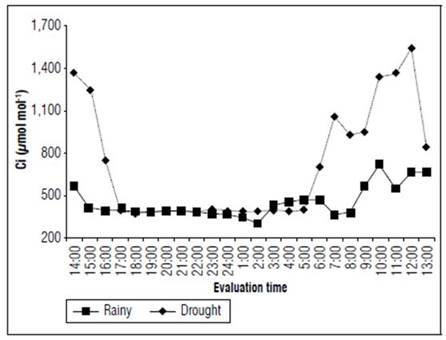
Figure 6 Internal CO2 concentration (Ci) in the forage cactus Mexican Elephant Ear [Opuntia stricta (Haw)] at different evaluation times during the rainy and dry seasons.
In both periods of the year, but mainly in the dry season, Ci was high in the first hours of the morning, followed by decreases throughout the afternoon. This could possibly be explained by the uptake of CO2 during the night, resulting in malate (malic acid) formation in vacuoles, followed by decarboxylation for CO2 fixation as a usual process in the plants (Sampaio, 2005). Although the lowest stomatal conductance was observed during the dry season, the Ci was higher during the rainy season. This means that the forage cactus maintained its physiological functions even under water shortage conditions (Fig. 3 and 6) and was probably able to accumulate gasses because of the interference of a non-stomatal factor (Larcher, 2006).
In the rainy season, the instantaneous water-use efficiency (Fig. 7) reached a value of 28.1 μmol CO2 m-2 s-1at 2:00 for each mmol H2O m-2 s-1lost in transpiration. On the other hand, in the dry season, the peak (2.21 μmol CO2 -2 s-1/mmol H2O m-2 s-1) was reached at 1:00 In the dry season, the iWUE values were low and stable. Furthermore, the values were positive during the entire day.
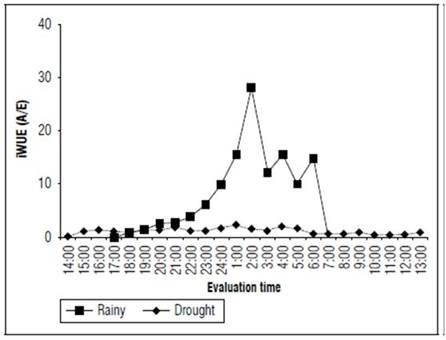
Figure 7. Instantaneous water-use efficiency (A/E) of the forage cactus Mexican Elephant Ear [Opuntia stricta (Haw)] at different evaluation times during the rainy and dry seasons.
A high water-use efficiency is one of the main advantages in cultivating xerophyte plants, especially succulents with the CAM mechanism since their potential in dealing with water scarcity substantially overcomes the potential of ones with the C3 and C4 metabolism. Nunes et al. (2017) obtained a WUE of 3.96 μmol CO2 m-2 s-1/mol H2O when cultivating passion fruit (Passiflora edulis Sims) with an irrigated cropping system. When assessing maize cultivation (Zea mays L.) in different sowing periods, Santos et al. (2018) verified a WUE of 3.85 μmol CO2 m-2 s-1/mol H2O m-2 s-1. For the forage cactus, the results showed that this cactus can obtain a WUE that is up to 7.1 times higher than passion fruit plants (C3) and 7.3 times higher than maize plants (C4).
When the intrinsic water-use efficiency (WUE) was compared at 2:00 in the rainy (88.5 μmol CO2 m-2 s-1/mol H2O m-2 s-1) and dry seasons (7 μmol CO2 m-2 s-1/ mol H2O m-2 s-1). Similar to the WUE during the rainy season, the WUE data were highly variable; they reached average minimum and maximum value of -36.6 and 88.5 μmol CO2 m-2 s-1/ mol H2O m-2 s-1, respectively. In the dry season, the fluctuations were between 0.0 and 10.1 μmol CO2 m-2 s-1/ mol H2O m-2 s-1 (Fig. 8).
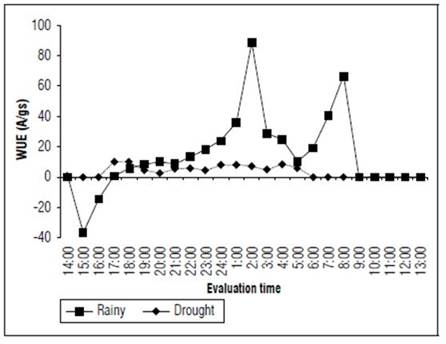
Figure 8. Intrinsic water-use efficiency (A/gs) of the forage cactus Mexican Elephant Ear [Opuntia stricta (Haw)] at different evaluation times during the rainy and dry seasons.
According to Bertolli et al. (2015), the increase in the WUE indicated that the net photosynthetic rate was elevated even with a low stomatal conductance, meaning that the decrease in gs was relatively more important than the decrease in A, which supports the presence of a stomatal limitation.
Despite the high values of these results, they were lower than the ones found by Silva et al. (2013) in research on sugarcane (Saccharum officinarumL.) genotypes and the ones obtained by Jacinto Júnior et al. (2019) when working with lima bean (Phaseolus lunatus L.) genotypes under extreme water shortage conditions. These authors explained that these high values were the result of losing water more intensely before photosynthesis inhibition takes place. These findings highlight the fact that, when stomata are open and there is water loss via transpiration, the plant is able to uptake carbon by producing photo-assimilated compounds.
Therefore, considering these results, it is possible to confirm the importance of studying gas exchange processes in the forage cactus. Recent research has a direct relationship with crop growth and with the possibility of better understanding the dynamics of the CAM mechanism in different periods of the year, certainly providing theoretical support for further research.
CONCLUSION
The gas exchange in the forage cactus Mexican Elephant Ear was significantly intense during the rainy season. In the dry season, it was stable, even though at low levels.
Regardless of the period of the year, the uptake of CO2 peaked between 24:00 and 2:00, indeed, this time range was the most suitable for analyses that accurately represented the field conditions.





![In vitro effect of cocoa leachates ongrowthanddevelopmentof Moniliophthora roreri ([Cif.] H.C. Evans et al.) isolatedfrom Theobroma cacao (L.)](/img/en/next.gif)