INTRODUCTION
Tomato (Lycopersicum esculentum L.) is a perennial plant often cultivated as an annual, with a bush-like growth pattern. It presents high genetic diversity, with countless varieties differing in appearance, color, and taste. This crop continues to experience increasing demand worldwide, underlining its importance in production and commercialization (Waheed et al., 2020). Tomatoes thrive in warm to moderate climates but are susceptible to frost and low temperatures. They can be cultivated in greenhouses or outdoors, needing good soil drainage and high organic matter content. However, if exposed to excessively low or high temperatures, the plant progressively weakens, potentially leading to death (Alarcon et al., 2020).
In Colombia, tomato production is commonplace across the country but is primarily concentrated in Antioquia, Norte de Santander, Valle de Cauca, Boyaca, Huila, Cundinamarca, Risaralda, and Caldas departments, spanning approximately 15,849 ha (Sepúlveda-Flórez, 2016). However, tomato cultivation faces significant challenges from various pests and diseases, including physiological disorders not caused by pathogens or insects, resulting in an increasing reliance on agrochemicals and affected yields (Panno et al., 2021). One such notable disease is anthracnose, a fungal disease causing substantial yield loss and significant damage to fruits (Shahriar et al., 2023). The disease symptoms can be present on fruits, stems, and branches at different phenological stages, particularly during rainy periods with average temperatures between 13 to 15ºC and 95% environmental humidity.
Anthracnose in Colombia has been reported to be caused by the Colletotrichum acutatum, C. boninense, and C. gloeosporioides, based on their morphological characterization. Although traditional methods to mitigate, control, and eradicate Colletotrichum spp. populations are employed, and the use of chemical compounds such as Benomil50® (AgroIQC) poses environmental and health issues and affects microbial diversity. In recent years, bioactive organic compounds extracted from plants have been used to control pathogenic microorganisms due to their antifungal properties, thus reducing the environmental impact of fumigation (Kong et al., 2020). Anthracnose, causes typical necrotic lesions on the stems, leaves, and fruits, has a considerable relevance in agriculture. It poses the potential to destroy up to 100% of the harvest and frequently results in chronic yield reduction (Ciofini et al., 2022). This phytopathology, caused by Colletotrichum spp., is one of the main constraints in tomato cultivation and often occurs during rainy seasons and under high relative humidity conditions, damaging leaves and fruits at any phenological stage, and also in postharvest (Ciofini et al., 2022).
Fungal control is typically carried out with chemical products that can be highly toxic and contribute to environmental deterioration and subsequently poor fruit quality. Owing to the buildup of disease resistance from the overuse of synthetic fungicides, there is a growing recommendation for alternative disease management strategies (Percival and Graham, 2021). Recent research focuses on evaluating alternative controls, such as antagonist microorganisms, physical treatments, and natural substances like essential oils and different types of extracts to reduce dependence on these fungicides (El Khetabi et al., 2022). Phytopathogen control with plant extracts is mainly due to the presence of secondary metabolites synthesized in plants as part of their defense mechanism. The diversity of components in the extracts explains their broad spectrum of biological activity (Elhamouly et al., 2022).
Plant extracts from several Cnidoscolus species have been reported to disrupt the biological cycles of pests and diseases caused by fungi and bacteria in various crops. Cnidoscolus urens L., a species with significant nutritional and medicinal potential, belongs to the Euphorbiaceae family and exhibits a biochemical composition based on anthocyanins, anthraquinones, coumarins, flavonoids, steroids, lignans, saponins, tannins, terpenoids, xanthines, and alkaloids (Carvalho Neto et al., 2018). This plant has attracted the attention of the scientific world, primarily due to the multiple biological actions it can perform. Research involving plant extracts as medicine is of increasing interest due to the progressive information on the antimicrobial activity of crude extracts (Chaves-Bedoya, 2022).
In the contemporary agricultural landscape, the cultivation of table tomatoes (Lycopersicum esculentum) faces significant threats from the phytopathogenic fungus Colletotrichum spp., which is responsible for anthracnose, a disease causing substantial economic losses regionally. Given this scenario, the search for new, sustainable, and environmentally benign alternatives to control this pathogen becomes imperative. In this context, our research spotlighted plant extracts from the medicinal plant C. urens as a promising contender, building on its reported potent biological activity against a variety of microorganisms.
The aim of this study was to evaluate the in vitro effect of ethanol extracts of C. urens L. on Colletotrichum spp. primarily responsible for postharvest anthracnose disease in tomato crops. This study favors sustainable development and the use of the region's natural resources, offering alternatives to be incorporated into agroecological management to reduce the use of chemical fungicides in combating diseases in agricultural production.
MATERIALS AND METHODS
Collection of Cnidoscolus urens L.
Vegetal material (leaves and stems) of Cnidoscolus urens L. was collected in the municipality of Cucuta (Norte de Santander-Colombia), at coordinates 7°52'56.4" N 72°33'06.2" W. Each sample was transported to the research laboratory Fitobiomol and stored at -80°C until the moment of processing the samples (Fig. 1).

Photos: Giovanni Chaves-Bedoya
Figure. 1. Collection and preparation process for the ethanol extract of C. urens (commonly known as “pringamosa”). (A) Milky latex of C. urens on the stem, with visible trichomes. (B) Samples stored in paper bags; note that collectors use gloves to prevent irritation from the stinging trichomes. (C) Leaves and flower of C. urens.
Preparation of ethanolic extracts
Dry vegetative material from C. urens was used, stored in envelopes containing 100 g of each sample and kept in a freezer at -20ºC for subsequent lyophilization. This process involves removing water from the vegetative material through freezing. After 24 h of lyophilization, each sample of the dried, crushed vegetative material was ground and weighed. A 96% ethanol solution (Merck, Germany) was added at a 1:6 weight/volume. The samples were then agitated for 24 h in total darkness on a shaker (MAXQ 4450, Thermo Scientific TM. Marietta, USA) at 29ºC and 150 rpm. The initial sample 1 (leaves) after being lyophilized and ground weighed 19.5 g and the initial sample 2 (stems) weighed 15.6 g; this weight was brought to a 1:6 weight/volume with 96% ethanol. The obtained extract was vacuum filtered using filter paper (Qual. Dia. 125 mm, BOECO, Germany) with the help of a funnel and vacuum pump (DOSIVAC, Buenos Aires, Argentina). Subsequently, the ethanol extract of C. urens was concentrated under reduced pressure in a rotary evaporator (IKA®RV10, Wilmington, USA) at 50 rpm with 150 mbar and 40ºC. The concentrated extract was stored in amber jars at 4°C for later antifungal activity testing against Colletotrichum spp.
Isolation and purification of Colletotrichum spp. strain
The fungus Colletotrichum spp. was isolated from a tomato fruit samples (Lycopersicum esculentum) exhibiting anthracnose symptoms (Fig. 2).
The fruits were sourced from the “Abastos” central market in Cucuta. The tomato samples were disinfected with 2% sodium hypochlorite for 5 min and subsequently rinsed with 70% ethanol for 3 min. The fruit explants were plated in triplicate on PDA (Merck) medium and incubated for 72 h at a temperature of 28°C. Upon observing fungal growth on the PDA medium, a single conidium was isolated under a microscope using a sterilized inoculation loop. This conidium was transferred to a fresh PDA plate to allow its growth. Once the conidium germinated and grew, the resulting colony was confirmed to have originated from a single conidium, ensuring a monoconidial culture. Successive re-plating was performed to further ensure a pure strain of the fungus of interest.
Molecular identification of isolated and purified strains of Colletotrichum spp.
For the extraction of fungal DNA, two purified strains of the fungus were utilized. Our extraction protocol was adapted and based on the methodologies described previously (Martínez-Culebras, 1999; Álvarez et al., 2004). The verification of Colletotrichum spp. genomic DNA was carried out using a 1% agarose gel, where the extracted genetic material from the C1 and C2 samples, corresponding to the fungal strains, was observed. DNA amplification was performed in a LTCG-48-101 gradient thermocycler (Labocon, Leicester, UK) with a final reaction volume of 25 µL, using ITS 3 (5'-GCATCGATGAAGAACGCACC-3') and ITS 4 (5'-TCCTCCGCTTATTGATATGC-3') oligonucleotides. The reaction mixture was prepared using Biolobas® Taq polymerase (Ipswichj, MA), following the manufacturer's instructions. Denaturation began with a temperature parameter of 95°C for 5 min, followed by denaturation at 95°C for 30 s, hybridization at a temperature of 52°C for 30 s, and final extension at 68°C for 2 min in 35 cycles.
The verification of the amplified products was carried out on 1% agarose gels, which were subsequently stained with Gelred® (Biotium, San Francisco, CA). The PCR product obtained was purified using the Bioline's Isolate II PCR and Gel Kit. The purified product was sent to Macrogen for sequencing. The nucleotide sequence obtained was compared with a search for homologous sequences using the BLAST algorithm, in the gene bank database of the NCBI (Benson et al., 2005).
Identification of secondary metabolites by gas chromatography-mass spectrometry (GC-MS)
The ethanolic extracts of C. urens (leaves and stems) were submitted to a specialized service for GC-MS analysis. While the exact sample preparation procedure is proprietary to the service provider, it generally involves derivatization or other sample preparation techniques to enhance the detectability of specific compounds such as fatty acids.
The chromatographic analysis was carried out using an AT6890 Plus series gas chromatograph equipped with an MSD 5973 mass spectrometric detector (Agilent Technologies, Santa Clara, USA) in full scan mode. The analysis utilized a DB-5MS column (5% phenyl-polymethylsiloxane, 60 m × 0.23 mm × 0.25 μm). The injection was executed in Split mode (30:1) with the use of a Solid Phase Microextraction (SPME) device.
The identification of the compounds was facilitated by the Adams, Wiley, and NIST databases. Specific details on the detection algorithm or software used in the NIST database comparison might be proprietary to the service provider.
Experimental design
The experimental design was structured around two experimental groups, each comprising eight treatments. These treatments involved dilutions of the extracts (extract-ethanol), a negative control using the antifungal agent Vitavax at a concentration of 24 mg mL-1, and a positive control with the PDA medium devoid of any added treatments. Each treatment was replicated in triplicate.
The primary variable measured during the study was the diameter of the fungal growth. Data analysis was conducted using SAS v 8, employing analysis of variance (ANOVA) to determine the significance of the observed differences among treatments. After observing significant differences in the ANOVA, multiple comparisons were conducted using Tukey's post hoc test to pinpoint specific differences between individual treatment groups.
The culmination of the experiment was marked by the complete growth of the fungal mycelium in the positive control Petri dish (Tab. 1).
Table 1. Summary of treatments using various dilutions of C. urens extracts. The dilutions are specified in the format 'extract:ethanol'. Vitavax fungicide was used as a positive control.
| Treatment | Description |
|---|---|
| -Control | PDA - negative control |
| +Control | PDA + positive control Vitavax |
| 1:0 | PDA + Dilution 1:0 |
| 1:1 | PDA + Dilution 1:1 |
| 1:2 | PDA + Dilution 1:2 |
| 1:3 | PDA + Dilution 1:3 |
| 1:4 | PDA + Dilution 1:4 |
| 1:5 | PDA + Dilution 1:5 |
RESULTS
Macroscopic and microscopic identification of Colletotrichum spp.
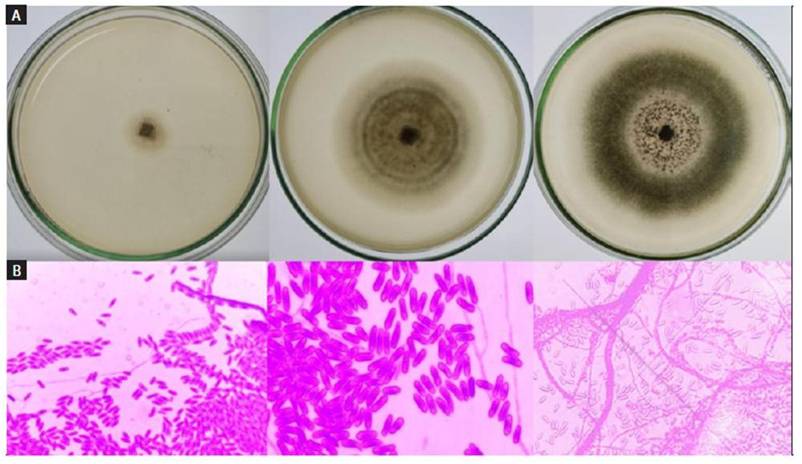
Once the disinfection and sowing process of tomato explants showing symptoms of Colletotrichum infection was carried out, the macroscopic and microscopic identification of the fungus was performed using the lactophenol cotton blue staining technique. This method was used for the characterization of conidia and fungal mycelium with the aim of identifying the Colletotrichum spp. Genus, using Barnett and Hunter´s key (Barnett and Hunter, 1986). The results provide substantial evidence that the fungus' growth observed on the culture plate, alongside the microscopic features of the hyphae and conidia, strongly corroborate the identification of the isolated fungus as Colletotrichum spp. (Fig. 3).
Molecular identification of Colletotrichum spp.
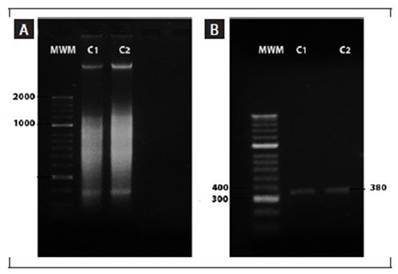
The amplification of the genomic DNA of the fungus via PCR yielded a band approximately 380 bp in size (Fig. 4). Comparative analysis of the amplified DNA sequence from strain C1 corresponds to the ITS 1, 5.8S ribosomal gene, internal transcribed spacer 2, and the partial sequence of the 28S ribosomal gene across various isolates of Colletotrichum spp. The sequence from strain C1 displayed a 99.36% similarity and 98% coverage with Colletotrichum boninense, presenting an E-value of 2e-155. However, no sequence was obtained from the C2 isolate, possibly due to inadequate purification of the DNA segment.
Main compounds in the ethanolic extracts of C. urens
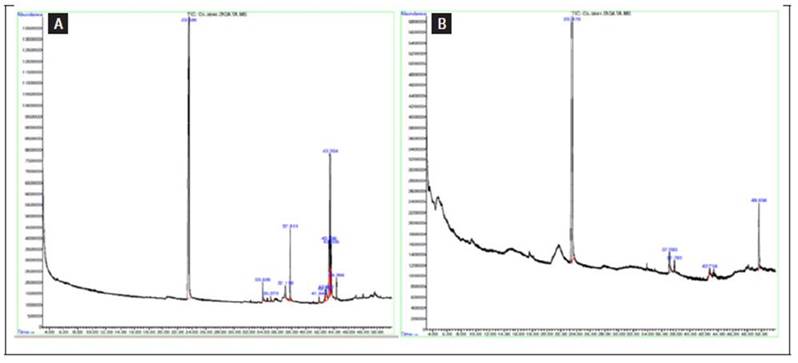
From the GC/MS analysis, several compounds were identified in the ethanolic extracts from the leaves and stems of C. urens. Among these compounds identified in the leaf extract are n-hexadecanoic acid, nonadecanoic acid, ethyl ester, phytol, and 9,12-octadecadienoic acid, ethyl ester. The stem extract analysis revealed the presence of certain fatty acids and triterpenoids. It's important to note that these are not the only components, but those detected by our GC/MS analysis. Further analyses, such as HPLC, could reveal additional compounds that might also contribute to the antifungal activity (Tab. 2).
Table 2. Compounds identified by GC-MS analysis in the ethanol extract (leaves and stem) of C urens L.
| TR, (Min) | Compounds | Relative amount GC |
|---|---|---|
| Leaves | ||
| 37,117 | n-Hexadecanoic acid | 99% |
| 37,8133 | Nonadecanoic acid, ethyl ester | 94% |
| 41,8446 | Phytol | 76% |
| 43,2457 | 9,12-Octadecadienoic acid, ethyl ester | 99% |
| Stem | ||
| 37,091 | n-Hexadecanoic acid | 97% |
| 49,6577 | Squalene | 91% |
This group of compounds has previously been identified with various biological properties, highlighting antimicrobial characteristics. n-Hexadecanoic acid has been cataloged as a defense to prevent infections. Similarly, the presence of phytol, a diterpenoid, has been highlighted as potential for a wide range of applications in the pharmaceutical and biotechnological industry.
Further insight into the chemical constituents of the ethanolic extracts from C. urens can be gleaned from the chromatograms presented. For a detailed visual representation of the peaks corresponding to the primary metabolites identified in both the leaves and stems, refer to figure 5.
Efficacy of C. urens ethanolic extracts against Colletotrichum spp.
Both the ethanolic leaf and stem extracts of C. urens were evaluated for their antifungal activity against Colletotrichum spp. at six different dilutions (1:0 to 1:5). The controls used for the experiment were water as a negative control, indicating normal fungal growth, and the antifungal Vitavax (+control), indicative of inhibited fungal growth. Remarkably, both the leaf and stem extracts (Fig. 6) displayed equivalent antifungal activity to the +control, successfully inhibiting the growth of Colletotrichum spp. In stark contrast, the fungus experienced normal growth in the presence of the -control, confirming the antifungal activity of the C. urens extracts (Fig. 6).

Figure 6. Inhibitory effect of C. urens leaf and stem extracts against Colletotrichum spp. The figure illustrates the inhibitory effects of both leaf and stem extracts of C. urens on the growth of Colletotrichum spp. In each respective petri dish, it shows a comparison between the control growth and the inhibited fungal growth following the application of the extracts. The differences in fungal growth between the control and extract-treated dishes highlight the potential of C. urens extracts as a natural fungicide. T1 negative control, T2 positive control (Vitavax), T3 dilution 1:0, T4 dilution 1:1, T5 dilution 1:2, T6 dilution 1:3, T7 dilution 1:4, T8 dilution 1:5.
DISCUSSION
Colletotrichum spp. is a ubiquitous phytopathogenic fungus that inflicts disease across a wide range of plant species, notably impacting productivity and fruit quality. Given the rising demand for sustainable and environmentally friendly control strategies, this study explored the potential of natural plant extracts as antifungal agents. We specifically examined C. urens, a plant species that has demonstrated potential in earlier studies (Chaves-Bedoya and Ortiz-Rojas, 2022). Its effectiveness against Colletotrichum spp. was evaluated using various concentrations of leaf and stem extracts.
An analysis of variance (ANOVA) was conducted to assess the impact of C. urens leaf and stem extracts on inhibiting Colletotrichum spp. growth. Several factors were contemplated in this study, which included the type of extract (leaf or stem), the extract concentration, and the repetition of the experiment. The analysis disclosed that both the type of extract and its concentration significantly affected the inhibition of fungal mycelial growth.
According to the ANOVA outcomes, C. urens extracts are successful in stymieing the growth of Colletotrichum, and this efficacy does not show any significant fluctuation between leaf and stem extracts or across various extract concentrations. The GC-MS analysis of the ethanolic extracts unveiled the presence of secondary metabolites, including terpenoid-type compounds. These constitute the most significant class of natural products used across diverse sectors like the food industry, pharmaceuticals, chemicals, and lately, they have also been exploited in the development of biofuel products. The presence of these critical compounds makes plants a valuable source for the discovery of novel products with medicinal value and drug development (Chaves-Bedoya and Ortiz-Rojas, 2022).
Recent studies have demonstrated the effectiveness of certain terpenes such as phytol, which showcases antimicrobial properties, specifically an antifungal effect against Candida spp. from the vegetable extract of Syzygium cumini (L.) (Figueiredo et al., 2021). Also, numerous investigations have verified the antimicrobial capability of fatty acids such as palmitic acid (n-hexadecanoic acid) and its derivatives, which are deemed as the most potent defense mechanism of certain organisms to prevent infections. Palmitic acid has shown significant inhibition results against various Candida spp. species (Quiroz-Lobo et al., 2022). Based on these findings, the major compounds identified in the ethanolic extracts of C. urens could potentially contribute to the biological activity against the phytopathogen Colletotrichum spp.
The results show that the vegetable extract dilutions from C. urens, both from leaves and stems, effectively inhibited the growth of Colletotrichum spp. across all tested concentrations from treatment 1:0 to treatment 1:5. The fungal radial growth, measured from the center of the inoculum to the growing edge, was only 1 mm under these conditions, compared to the 55 mm observed in the positive control corresponding to treatment negative control. This significant finding suggests that these extracts may harbor potent antifungal properties. In other words, no significant differences were observed between the efficacy of leaf and stem extracts based on these data.These findings support the idea that C. urens extracts could serve as an effective biological antifungal alternative or plant protection agent for controlling fungal diseases across diverse plant species. This indicates that the antifungal compounds present in this plant could be distributed both in leaves and stems, a valuable observation for future investigations of these extracts as potential antifungal agents.
Compared to the positive control (Vitavax), the extracts showed similar efficacy in restraining the mycelial growth of the fungus to 1 mm. This is encouraging as it suggests that C. urens extracts could be as potent as existing commercial fungicides. With regard to the efficacy of different dilutions, there was no significant variation in the ability to inhibit fungal growth across multiple extract concentrations. Nevertheless, conducting further tests would help confirm these results and identify if an optimal concentration exists. Further, it is crucial to identify the specific antifungal compounds in the extracts and investigate their potential applications in practical scenarios, such as treating plant fungal diseases.
In addition to the major compounds identified in the ethanolic extracts of C. urens, it is critical to note the potential of unexplored or unidentified compounds that may contribute to the observed antifungal activity. The synergistic effect of various compounds could play a pivotal role in enhancing the efficacy against Colletotrichum spp. For instance, recent studies have highlighted the importance of plant synergism where a combination of compounds can result in enhanced antimicrobial activity (Vaou et al., 2022).
Moreover, the potential of these extracts extends beyond being merely antifungal agents. Plant-based extracts have been documented for their antioxidative, anti-inflammatory, anticancer, and various other medicinal properties (Yuan et al., 2016). The presence of secondary metabolites like terpenes and fatty acids in the extracts from C. urens could potentially have similar multi-faceted health and medicinal benefits, thus warranting further research in this direction.
Finally, while our findings suggest promising potential of C. urens extracts in controlling fungal growth, it is also essential to consider the practicality and implications of these findings. Factors like the availability and growth rate of C. urens, the scalability of extract production, and potential environmental impacts of large-scale implementation need to be carefully evaluated. The safety and potential side effects, especially if applied in a food crop context, must also be considered. In essence, while laboratory results are indeed promising, translating these findings into effective, safe, and environmentally friendly solutions requires comprehensive multidisciplinary research.
Therefore, the results of this study not only underscore the potential use of C. urens extracts in managing phytopathogenic fungi but also open avenues for broader explorations into their biochemical properties and potential applications in diverse sectors, including medicine, agriculture, and the food industry.
CONCLUSION
Through this study, we not only accomplished the morphological and molecular characterization of the Colletotrichum spp. fungus isolated from Lycopersicum esculentum fruits with disease symptoms, but we also shed light on the in vitro efficacy of C. urens extracts. Our observations suggest that all tested concentrations of these extracts were as potent as the commercial fungicide Vitavax in inhibiting the mycelial growth of Colletotrichum spp.
We further discerned the presence of key compounds such as terpenes and fatty acids in the extracts, which we posit, could be instrumental in the antifungal properties exhibited. Our findings thus bolster the hypothesis that C. urens extracts could serve as a viable and sustainable alternative to commercial fungicides for managing Colletotrichum spp. growth.
While our research affirms the antifungal potential of C. urens extracts, it also opens avenues for future studies. Deeper investigations could focus on identifying the precise antifungal compounds and understanding their mechanisms of action. Furthermore, field studies would be beneficial to test the practical applicability and environmental impact of using these plant extracts as bio-fungicides. Our study underscores the importance of embracing eco-friendly approaches to agriculture, fostering a balance between productivity and sustainability.