INTRODUCTION
The phytocannabinoids Δ9-tetrahydrocannabinol (THC) with psychoactive properties and Cannabidiol (CBD) that are used in the treatment of pathologies such as epilepsy and fibromyalgia are the main phenolic compounds synthesized in the globular trichomes in the female inflorescences of the cannabis plant (Cannabis sativa L.) (De Backer et al., 2012; Wróbel et al., 2022), an ancestral plant whose uses date back to antiquity (Molina, 2008; Clarke and Merlin, 2013) and which has now gained importance in medicinal use, thanks to the discovery of the endocannabinoid system in mammals (Grotenhermen, 2006).
Cannabis is a mainly dioecious plant, where the female and male inflorescences are found on separate individuals, considered an allogamous and cross-pollinated species favored mainly by wind (Small et al., 2003; Caplan et al., 2018). Although the plant can be propagated sexually and asexually, the progeny derived from the seeds can show high segregation. This condition makes it difficult to maintain uniformity both in the architecture of the plant and in the phytochemical content and quality of the active ingredients (Coffman and Gentner, 1979; Chandra et al., 2017). For this reason, asexual propagation methods such as cloning and tissue culture have been used to maintain both the morphological and phytochemical characteristics of the individual as well as culture uniformity (Chandra et al., 2017; Caplan et al., 2018; Kodym and Leeb, 2019). Cloning is a propagation technique widely used by flower producers in Colombia, a crop that represents an important line in the country's economy (Chica et al., 2016). However, although this technique is perfectly adapted to cannabis, it is necessary to adjust the multiplication protocol to be taken to an industrial scale (Cervantes, 2007; Chandra et al., 2017; Caplan et al., 2018).
Several factors favor the rooting of cannabis cuttings, including the number of leaves and size of the cutting. Wahby (2007) and Caplan et al. (2018) obtained the best results when the cutting kept 2 to 3 fully expanded leaves. Likewise, the use of auxins such as indolebutyric acid (IAB) and naphthaleneacetic acid (ANA) has been documented as a stimulant in root formation in asexual propagation methods of different species, including cannabis (Hernández et al., 2005; Cervantes, 2007). Another of the practices to which reference is made is the removal of at least one or two-thirds of the length of the leaflets in order to reduce the leaf area and transpiration and avoid the appearance of diseases due to the density of the biomass in the propagation chambers; however, research by Caplan et al. (2018) report a decrease concerning the formation of roots of the cutting under this practice.
Based on the above, cannabis growers perform some of these practices in the cutting as a strategy to increase rooting success; however, there are few scientifically validated protocols focused on the production of clones using this method. The present study aims to evaluate asexual propagation techniques as an alternative to the massive production of cuttings that allows for maintaining a permanent demand for seedlings for cultivation.
MATERIALS AND METHODS
Location and collection of material: This work was carried out at the Breeder's de Colombia S.A.S., facilities registered with the Colombian Agricultural Institute (ICA in Spanish) as a research unit in improving cannabis, according to resolution No. 00030034 of August 14, 2018, and located in the municipality of Bello (Colombia).
Mother plants: Cannabis seeds of the Candida CD-1 variety were planted in 30-L containers with a substrate composed of a mixture of equal proportions of coconut, rice scoop, mushroom, and sawdust. The plants were located under a plastic cover, with a photoperiod of 18 light hours, of which 12 h is natural light and the remaining 8 h supplemented with the help of 30 W and 5000 °K LED bulbs. The cuttings were taken from 6-month-old mother plants; branches from the last two-thirds of de mother plants were selected from cuttings with a length between 7 and 12 cm and three fully expanded leaves.
Experimental design: A completely randomized design was used, with eight treatments and 15 repetitions, in a 2×2×2 factorial arrangement. Factor a corresponded to the sowing medium (peat soil and hydroponic), factor b to the shoot tip management (with and without tipping), and factor c to the use of a growth regulator (with and without regulator).
In the case of the sowing media (SM), two types of techniques were used. The first is a commercial substrate based on peat soil (Forza Mix®, Fercon, Yumbo, Colombia) in germination trays with alveoli dimensions of 5×5 cm. The peat soil was previously moistened with water before planting the cuttings. The other method was based on a hydroponic system powered by a submersible pump and a micro-sprinkler system to disperse the water inside a closed container. In this method, the pH of the solution was maintained between 5.5 and 6.3. Daily corrections were made with HCl and NaOH as required, and the electrical conductivity (EC) was at 0.5 mS cm-1 with the addition of water-soluble salts (Hidroinicio® 13-40-13 + minor elements, Tierraagro, Medellin, Colombia). For the shoot tip management (TM) factor, a third of the leaflets were cut from each fully expanded leaf. Finally, the growth-regulating factor (GR) consisted of immersion of the base (3 cm) of the cuttings in a medium composed of 0.40% naphthaleneacetic acid (Hormonagro1®,Colinagro, Bogota). For cuttings without growth-regulating treatment, the base of the cutting was submerged in deionized water. A temperature of 25°C±3, a photoperiod of 18 light hours (5W and 5000K LED lamps), and a photosynthetic photon flux density (PPFD) of 58 µmol photons m-2 s-1 were maintained. The PPFD was measured using a daily integral and photoperiod meter Apogee DLI-500 (Logan, UT, USA). Relative humidity was above 95% for the first ten days and gradually decreased until it reached 75% on day 17, where the cuttings were harvested.
Evaluated variables: The variables evaluated were cutting survival rates (%) as the proportion of living cuttings with or without root formation about the number of cuttings planted; the rooting success - (%) as the relationship between cuttings with visible roots to the total number of cuttings planted. Other variables evaluated were the number, length, and volume of the roots formed. Root length was estimated from the ImageJ software, whereas the root volume was determined as the displaced volume of a liquid inside a 10 mL graduated Beaker. The dry matter was determined after placing the samples in drying ovens (Memmert type UL 80) at 70°C until reaching a constant weight.
Statistical analysis: A variance analysis was performed after validating the assumptions of normality and homoscedasticity (Shapiro-Wilk and Bartlett tests). Subsequently, the variables that presented a P-value <0.05 in the ANOVA were submitted for the Tukey (P=0.05) multiple means comparisons test using the Agricolae package of the R statistical software Core Team 2023.
RESULTS AND DISCUSSION
The ANOVA, P values for the evaluated variables are shown in table 1. Significance differences (P<0.05) were presented in the triple interaction (SM×TM×GR) for rooting, root number, and root length. In contrast, the variables root volume and dry root matter presented significant differences for the double interactions (TM×GR). For dry root matter and root volume, the SM×TM interaction also was significant. In the case of the survival variable, only the effect of the SM factor was significant.
Table 1. P-values for each factor evaluated in cannabis cuttings and for the double and triple interactions. MS: Sowing media, TM: Shooting tip management, and GR: growth-regulator.
| Variable | SM | TM | GR | SM×TM | SM×GR | TM×GR | SM×TM×GR |
|---|---|---|---|---|---|---|---|
| Survival rate (%) | 0.00074 | 0.27354 | 0.27354 | 0.71042 | 0.07704 | 0.27354 | 0.71042 |
| Rooting (%) | 0.05808 | 0.05808 | 1.58·10-10 | 7.08·10-05 | 0.05808 | 0.68850 | 0.00205 |
| Root number | 2.17·10-13 | 0.00476 | < 2·10-16 | 0.00326 | 3.41·10-12 | 0.01128 | 0.02197 |
| Root length (cm) | 0.02125 | 0.04853 | 0.13001 | 0.06744 | 5.32·10-09 | 0.77639 | 0.00099 |
| Root volume (cm3) | 0.5678 | 0.0113 | <2·10-16 | 0.0665 | 0.1386 | 0.0517 | 0.8328 |
| Root dry matter (g) | 0.1432 | 0.0183 | 2.00·10-16 | 0.0025 | 0.9298 | 0.1109 | 0.9324 |
Cutting survival rate: The cuttings' survival percentage presented a statistically significant difference for the DM factor (P=0.00074) (Tab. 1). The cuttings planted in peat soil presented the highest survival percentage, with 98.33%, which was 18.64% higher than the survival observed in the cuttings planted in a hydroponic medium (80%).
Rooting success - (%): The triple interaction MS×TM×GR was significant (P=0.0021). In this case, the percentage of rooting success is represented by graphs of double interactions (TM×GR) for each level of MS. The highest rate of cuttings with successful rooting occurred in hydroponic + without tipping + with a regulator (93.33%). In contrast, the lowest percentage was observed in cuttings planted in the combination peat + without tipping + without regulator (6.66%). Regarding the shoot tip management, no significant difference was observed between the cuttings planted in both media and received the hormonal treatment. Nevertheless, a decrease in rooting success was observed among cuttings planted in the hydroponic medium, subjected to hormonal treatment, and without apex tipping, about those who received apex cut-going from 93.33 to 80%. This was more marked and significant (P=0.0007) in cuttings planted in the same medium (hydroponic) without a growth regulator, which decreased by 60% for cuttings without apex management compared to 13% for those with an apex tipping (Fig. 1).
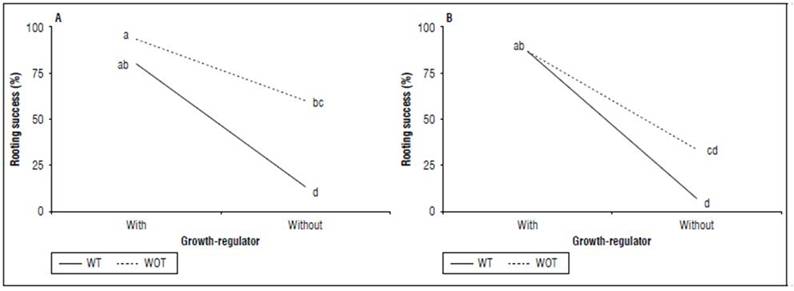
Figure 1. Interaction between shooting tip management (TM) by growth-regulator (GR) treatment for each planting medium for the rooting success (%) of cannabis cuttings. A, hydroponic. B, peat soil. Without (WOT) and with tipping (WT). Means with different letters indicate significantly different according to Tukey (P<0.05).
Root number: Like rooting success, the triple interaction MS×TM×GR was significant (P=0.0212), for which it is analyzed through the TM×GR interaction for each level of MS (Fig. 2). The hydroponic + without tipping + with a regulator combination found the highest number of roots (65.13) per cutting as long as the lowest value was registered in peat soil + without tipping + without growth regulator with 0.2 roots per cutting. The hydroponic + without tipping + growth regulator combination was significantly superior (P=0.0000) in this variable with 63.13 roots compared to the combination hydroponic + with tipping + growth regulator that reached 36.73 roots. In addition. the combinations peat soil + without tipping +growth regulator (P=0.0000) and peat soil + with tipping + growth regulator differed significantly for this variable (P=0.0000).
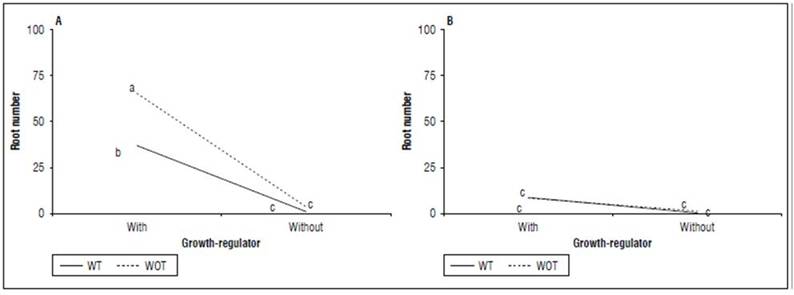
Figure 2. Interaction between shooting tip management (TM) by growth-regulator (GR) treatment for each planting medium for the root number of cannabis cuttings. A, hydroponic. B, peat soil. Without (WOT) and with tipping (WT). Means with different letters indicate significantly different according to Tukey (P<0.05).
Root length: The greatest lengths of the root were presented in peat soil + without tipping + growth-regulator, hydroponic + without tipping + growth-regulator, and peat soil + with tipping + growth-regulator with 3.46 2.92 and 2.45 cm, respectively. There was a significant difference between cuttings planted in peat and the application or not of hormonal treatment that were not submitted to the apex cut (P=0.0000). On the other hand, cannabis cuttings planted in hydroponics without hormones and tipping did not show significant differences with those planted in peat soil with growth regulators and without tipping the apex (Fig. 3).
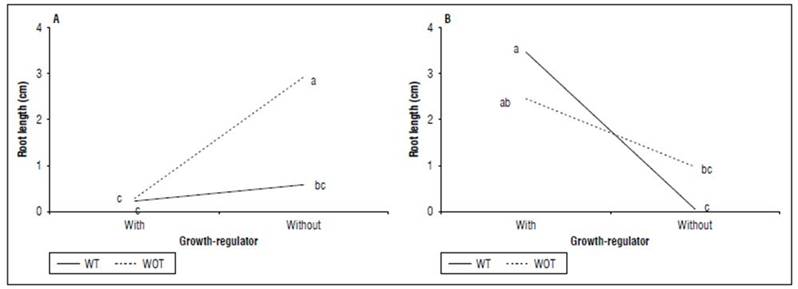
Figure 3. Interaction between shooting tip management (TM) by growth-regulator (GR) treatment for each planting medium for the root length (cm) of cannabis cuttings. A. Hydroponic. B. Peat soil. Without (WOT) and with tipping (WT). Means with different letters indicate significantly different according to Tukey (P<0.05).
Root volume: The cuttings with growth regulator showed the highest values in root volume (0.4133 mL) compared to cuttings that did not receive the growth regulator application (0.0348 mL), representing a significant effect for this variable (P=2.2·10-16) (Fig. 4). A similar case occurred with the TM factor, where cuttings without apex tipping showed greater root volume (0.656 mL) than those that were subjected to pruning (0.1825 mL), with significance between these two treatments (P=0.0113). Regarding the double interaction TM×GR (P=0.0517), cuttings without hormonal treatment showed the lowest values for root volume of 0.0250 mL for cutting and without a regulator and 0.0446 mL for cuttings without tipping + without growth regulator, without difference statistics between them (P=0.9731). Regarding cuttings with hormone application, the treatments without tipping + growth regulator (0.4866 mL) and with tipping + growth regulator (0.34 mL) presented a significant difference (P=0.0092) (Fig. 4).
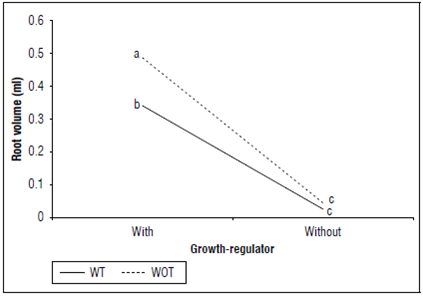
Figure 4. Interaction between growth-regulator (GR) by sowing media (SM) treatment for the root volume (mL) of cannabis cuttings. Without (WOT) and with tipping (WT). Means with different letters indicate significantly different according to Tukey (P<0.05).
Root dry matter: The simple effects of TM and GR showed significant differences with P= 0.0183 and <2·10-16, respectively. The highest root dry matter was obtained in the cuttings that kept their complete leaves (0.03092 g) about those that had their tips cut (0.0235 g); likewise, the cuttings subjected to the hormonal treatment (0.0481 g) exceeded in weight those not treated with the growth regulator (0.0062 g). The double TM×GR interaction was significant (P=0.0025), where the highest root dry matter was achieved in cuttings planted in a hydroponic medium without cutting the tips (0.0379 g) compared to cuttings that received the apex cutting (0.0209 g). (P=0.0010). The opposite occurred with cuttings planted in peat soil, which did not show significant differences between those subjected to cutting and those that received the apex treatment (P=0.9601) (Fig. 5).
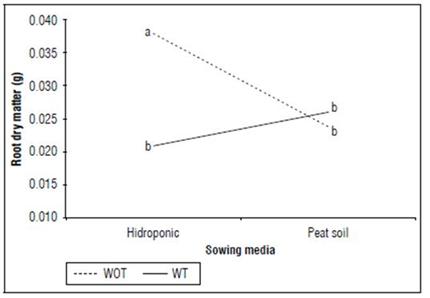
Figure 5. Interaction between shooting tip management (TM) by sowing media (SM) treatment for the root dry matter (g) of cannabis cuttings. Without (WOT) and with tipping (WT). Means with different letters indicate significantly different according to Tukey (P<0.05).
As a particular observation in this study, it is noteworthy that between the different sowing media (peat soil and hydroponic) and their possible combinations evaluated (TM and RG), the formation of callus cells was only evidenced in the cuttings that were planted in the peat soil and subjected to the hormonal treatment with ANA. This callous formation was present so much in the cuttings that the apex was cut like the untreated.
Cell division occurs mainly in meristematic tissues of the apices and the vascular cambium. For this reason, the apical tissues are preferred by growers for mass cloning, as this ensures maintaining homogeneity in cultures (Vassilevska-Ivanova, 2019). The high number of roots formed in cannabis cuttings in this study (Fig. 2) alludes to the ability of cannabis cuttings to regenerate root tissues through asexual reproduction under the cutting method. Studies conducted by Chandra et al. (2020) indicate that different culture media, such as soil and hydroponics, are efficient for root generation; nevertheless, when the cuttings are treated with a growth regulator (AIB), they initiate the formation of adventitious roots between the second and third week after planted in the culture media (soil and hydroponics). These results coincide with those reported in this study, where cannabis cuttings were planted in peat soil and hydroponics and subjected to the growth regulator with ANA, started the formation of roots from the second and third week after sowing; however, the cuttings that were not subjected to hormonal treatment, although their survival was high (100%), the percentage rooting success was lower than those treated with the regulator, this can be explained by the storage of nutrients and energy reserves within the healthy and vigorous cuttings. However, controlling environmental factors such as photoperiod, humidity, ambient temperature, and the substrate is critical to guarantee the cuttings' survival and entrenchment (Cockson et al., 2019; Chandra et al., 2020).
The action of auxins on cell expansion in plant growth has been extensively studied (Öpik et al., 2005). The application of hormonal inducers such as indole-3-butyric acid (IAB), indoleacetic acid (IAA), and 1-naphthaleneacetic acid (ANA) favor the formation of adventitious roots in cannabis cuttings (Caplan et al., 2018), reducing the time in the area of propagation as confirmed in the present study where cannabis cutting subjected to the hormonal treatment (ANA) issued 36.73 and 65.13 roots at 30 d after planting (Fig. 2), added to a greater success in rooting and root volume, but shorter root length, compared to cuttings without hormonal treatment (ANA), which lengthens the permanence time of the cuttings in the culture media, to obtain an adequate seedling as planting material. Lata et al. (2010) evaluated different concentrations and auxins such as ANA and in combination with thidiazuron (TDZ) for callus production from young cannabis leaves, reporting that the best results occurred in tissues treated with 0.5 µM of ANA added with 1.0 µM TDZ. As observed in the present study, cuttings planted in peat soil and subjected to treatment with naphthaleneacetic acid induced the formation of callus cells at the base of the cutting during cell differentiation processes. Although callus cell formation has been implemented as a tissue culture propagation technique, this cell formation can induce spontaneous mutations or somaclonalvariation of cuttings, as reported by Kodym and Leeb (2019), with its variations in the phytochemical components of the plant as indicated by Cox (2020), who measured the concentrations of different cannabinoids such as CBD and THC and their acid forms in industrial hemp plants that were subjected to successive propagation for three generations using the cutting method. The study demonstrated a decrease in the acid form of the phytocannabinoid cannabidiol (CBDA) in the second and third generations compared to the first for the Cherry×Workhorse variety. Similar data were reported in the Cherry variety, where there is a decrease in CBDA and an increase in THC levels in the second generation compared to the first. going from 0.0001 mg of THC/100 mg of flowers to 0.0008 mg of THC/100 mg.
Caplan et al. (2018) evaluated the effect of the cutting leaves number, the position in which it is located within the mother plant, the apex tipping, and the application of a hormonal regulator on the rooting success and quality of the roots of cuttings from cannabis stem. The results obtained in the present study coincide with those reported by the previous authors, which indicate that the best results, both in the rooting success and the quality of the roots, were observed in cuttings with a hormonal regulator based on IBA and kept complete leaf tips. Besides the fact that the auxin has been well known to act as a central regulator controlling adventitious rooting in plants. The primary auxin (indole-3-acetic acid - IAA) is synthesized in the young tissues of the shoot system (Ljung et al., 2002). Consequently, the adventitious or lateral root formation process comprises three successive phases: induction, initiation, and expression; Nevertheless, the critical molecular events occur during root induction and initiation phases. The auxin has been well known to act as a central regulator controlling adventitious rooting in plants and directly influences adventitious root formation, clearly playing a vital role in the initiation phase (Li, 2021). For this reason, exogenous auxin is sufficient to stimulate the formation of lateral roots throughout the pericycle cells adjacent to the xylem (Malamy, 2009).
The authors also indicate that the application of the synthetic hormone (IBA) achieved the greatest effect on the rooting rate of the cannabis cuttings, 2.1 times higher than the willow extract; likewise, the management of the apex showed the second most marked effect, where cuttings without apex tipping presented a 71% success rate concerning cuttings with apex tipping whose success was reduced to 53%. This may be because the leaves are a source of nutrient reserves and production of photoassimilates that support the cutting during root formation during rooting. The reduction of the leaf area, either through the excessive removal of leaves or the cutting of the apices, can affect the formation and quality of the roots due to the decrease in the sources of reserves; on the other hand, a greater quantity of leaves can favor the dehydration of the cutting by evapotranspiration through the stomata. Caplan et al. (2018) did not observe significant differences in the quality of the roots of cannabis cuttings with 2 and 3 fully developed leaves; however, cutting 30% of the apices does have a negative effect on this variable.
CONCLUSION
The asexual multiplication of cannabis by cutting is a technique that adapts to both the traditional peat-based method and hydroponic systems. Both planting media showed satisfactory results in the evaluated variables. Applying growth regulators such as ANA and maintaining three developed leaves and complete tips in the cuttings, either in hydroponic or in peat soil showed a rooting success above 80% and a 100% survival.




![Cultivation of Furcraea foetida ([L.] Haw.) under different nitrogen doses and sources](/img/en/prev.gif)










