INTRODUCTION
Cannabis sativa (L.) is a herbaceous, commonly dioecious, short-day annual plant grown worldwide for grain, fiber, and secondary metabolites (Campbell et al., 2021). It is a monospecific genus with two subspecies, C. sativa subsp. sativa and C. sativa subsp. indica, native to Europe and Asia, respectively (Rull, 2022). The geographical and ecological range of hemp is wide; it is found on all continents except Antarctica, in a wide variety of environments, from subarctic to temperate and tropical, and from sea level to more than 3,000 m altitude (Voeks, 2014; Lynch et al., 2016). In Colombia, various laws and decrees have supported the policy of using cannabis as a plant species for pharmaceutical purposes, under compliance with established regulations, and ratified a willingness to place the medical cannabis sector in a priority and strategic path for the development of the country, within the framework of legality and entrepreneurship (Araméndiz-Tatis et al., 2023).
Grain and fiber varieties that are planted exclusively by seed (Campbell et al., 2021) can generate high segregation due to genetic recombination, which makes it difficult to maintain homogeneity in crops (Lata et al., 2016). For this reason, the hemp that is used for pharmaceutical purposes spreads vegetatively, producing high contents of a group of secondary metabolites called phytocannabinoids (Busta et al., 2022). The main compounds are delta-9-tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), which by decarboxylation are transformed into tetrahydrocannabinol (THC) and cannabidiol (CBD) (Welling et al., 2016). These are mainly concentrated in the essential oils of the flowers of unfertilized female plants (Potter, 2014). Thus, male plants are not desirable within plantations as fertilized plants decrease the quality and concentration of fitocannabinoids (Trancoso et al., 2022). Asexual propagation by cuttings ensures genetic uniformity and production of female plants, making for a reliable and cost-effective method for essential oil production (Adhikary et al., 2021).
Within the process of asexual propagation of hemp plants, there are a number of factors that can affect the rooting of cuttings (Mejía-Londoño et al., 2023), such as genetics, age of the mother plant or the level of phytohormones (Campbell et al., 2021). Within the phytohormones, auxins play an essential role since they promote the initiation of adventitious root growth and root primordia through cell division as well as the hydrolysis of starch and the mobilization of sugars and nutrients to the cutting base (Sourati et al., 2022). Auxins such as indole-3-acetic acid (IAA), phenylacetic acid (PAA) and indole-3-butyric acid (IBA) are involved in the regulation of cell division processes, cell growth, and root development (Finet and Jaillais, 2012). However, cuttings, being small portions of the plant, usually have small amounts of intrinsic auxin, which limits the propagation by cuttings. This necessitates the exogenous application of synthetic auxins known as phytoregulators to improve rooting (Pizzatto et al., 2011).
Among the most used phytoregulators are indole-3-butyric acid (IBA), which generates a positive effect on the rooting processes of cuttings (Preece, 2003; Blythe et al., 2007, Pego et al., 2019), and synthetic auxin 1-naphthaleneacetic acid (NAA), which increase endogenous contents and induce physiological responses similar to natural auxins (Imin et al., 2005). Phytoregulators can commonly be applied to cuttings through liquid solutions or powder formulations, or a combination of both (Hartmann et al., 2014). Low concentrations have shown better hemp rooting success compared to auxin-free application (Caplan et al., 2018).
Indole-3-butyric acid (IBA) increases and accelerates rooting and root production and generates a significant improvement in root quality and seedling production (Abdel-Rahman et al., 2020). It is more stable than IAA and persists longer in plant tissues (Rastogi et al., 2013). For its part, 1-naphthaleneacetic acid (NAA) has been documented as a stimulant in root formation in asexual propagation methods in different species, including hemp (Campbell et al., 2019). Gil et al. (2020) indicate that the use of NAA allows cuttings to overcome the disadvantages caused by their low intrinsic auxin.
In line with the above and taking into account that in Colombia the area cultivated with hemp for pharmaceutical purposes has been increasing and that there is little information on the use of phytoregulators in the propagation process, the objective of the present study was to evaluate different doses of ANA and AIB in the rooting process of hemp cuttings cv. Eco-2 under greenhouse conditions.
MATERIALS AND METHODS
Location
The research was carried out on the farm La Chacra, owned by the company Clever Leaves, located in the municipality of Pesca (Colombia), 7°37" N and 73°00" W at an altitude of 2,527 m with an average temperature of 17°C and a relative humidity of 75%. The evaluation was carried out under greenhouse conditions. The average temperature inside the tunnel during the day was 22°C, with a relative humidity of 75%, and during the night was 13°C, with relative humidity of 85%.
Plant material
Hemp cuttings of the Eco-2 cultivar with a length of 10 cm and with three fully expanded leaves of mother plants were taken from the apical part of the plant, making a bevel cut. The mother plants were grown in soil, under a photoperiod of 18 h, with a light intensity of 750 μmol m-2 s-1, and were 14 weeks old at the time of the cutting.
Management of cuttings and experimental dosing
The effect of phytoregulators was evaluated using a completely random design with factorial arrangement 2×4, with the first factor the type of auxin phytoregulator, NAA or IBA and the second factor the doses, 0, 400, 800 or 1,000 mg L-1, with three replications, for a total of 24 experimental units. Each experimental unit consists of 10 cuttings each with a length of 8.5 cm and a diameter of 2.5 mm.
A p/v solution was prepared for each of the doses evaluated using 1-naphthaleneacetic acid (NAA) powder at a purity of 95% (Sigma Aldrich Chemical®) and Indole-3-butyric acid (IBA) powder at a purity of 98% (Sigma Aldrich Chemical®), with distilled water as solvent. 2 cm of the basal part of the cuttings was immersed in the hormonal solution with immersion for 15 s. For sowing, coconut pellets of 40×90 mm, hydrated before sowing, were used as substrate. These were arranged in rooting bank, conditioned with a fertigation system to guarantee the supply of irrigation and nutrients to the cuttings. The evaluations were carried out 4 weeks after the planting process.
Evaluation of morphological variables
Six quantitative variables were evaluated: cutting diameter (mm) by means of a Mitutoyo digital calibrator accuracy ± 0.05 mm, cutting length (cm) and root length (cm) by flexometer, number of new shoots expressed as number of buds with visible growth, root volume (cm3) by immersion in marked beaker to quantify volume displaced by the root section of each cutting, and percentage of rooting (%) expressed as the quotient between rooted cuttings and sown cuttings multiplied by 100.
Analysis of data
Statistical analysis of data was performed using statistical software R Core Team (2022). The data were subjected to tests of normality and homogeneity of variance using the Shapiro-Will and Barlett tests, respectively. Once the assumptions were verified, analysis of variance was performed; the variables that showed statistical differences were subjected to Tukey's mean comparison tests (P≤0.05) using the HSD function of the 'agricolae' package. The figures were made using the ggplot function of the 'ggplot2' package.
RESULTS AND DISCUSSION
Cutting length and diameter
The cuttings treated with auxin phytoregulators showed increased length with significant differences (P≤0.05). There was a positive response depending on the type of phytoregulatorand the dose applied. The longest length of the cuttings was observed when treated with a dose of 800 mg L-1 of NAA, which reached an average height of 12.3±0.25 cm, compared to the other treatments with application and compared to the treatment without application that presented the lowest average height with a value of 9.2±0.14 cm (Fig. 1A). The diameter of the cutting was not affected by the type of phytoregulator or the dose, with no statistical differences between treatments; the mean values for this variable ranged from 5.02±0.05 to 5.12±0.04 mm (Fig. 1B).
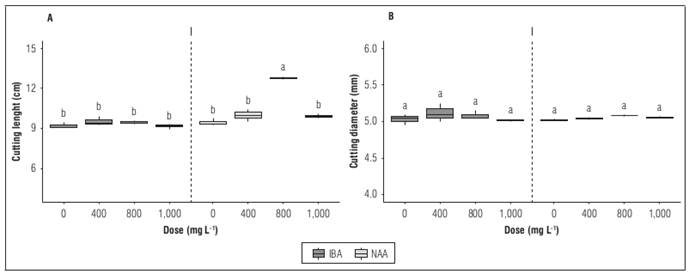
Figure 1. Growth behavior of hemp cuttings treated with different types of auxins phytoregulators applied in different doses: A, length of cutting; B, diameter of cutting. Means with different letters indicate significant differences according to Tukey's test (P<0.05).
Garay-Arroyo et al. (2014) state that auxins are hormones that are present throughout the life cycle of plants and are particularly interesting as they participate in different morphogenetic processes. They are usually very well distributed in most plant cells and tissues, which allows them to fulfill functions such as unicellular and multicellular differentiation or even act in different plant tissues (Alcantara et al., 2019; Bai et al., 2020). According to Borjas-Ventura et al. (2020), one of the main uses of auxins is to promote rooting, especially in low concentration.
The results showed that the application of NAA had no effect on diameter but did generate a greater length, with an increase of 33.9% compared to the treatment without application. Caplan et al. (2018) indicate that the application of phytoregulators such as 1-naphthaleneacetic acid (NAA) favors the growth of hemp cuttings, reducing the time in the propagation area. This generates greater commercial profitability and less greenhouse work.
It can also be observed that when applying a dose of 1,000 mg L-1 regardless of the phytoregulator used, a lower response was generated compared to the 800 mg L-1 treatment, which presented the best response. While the application of auxin phytoregulators can favor the process of propagation of cuttings in different plant species. Blythe et al. (2007) indicate that excessive concentrations of auxin can have a negative impact in the propagation process. Raya-González et al. (2018) show that the external application of auxins first affects the balance of endogenous hormones, which generates changes in the growth process of the shoots and/or the development of the roots.
Root length and volume
Hemp cuttings treated with auxin phytoregulators at different doses showed an increase in both root length and volume with significant differences between treatments (P<0.05). The NAA treatment at a dose of 800 mg L-1 presented the best values of both root length and volume, with mean values of 9.0±0.09 cm and 5.0±0.05 cm3, respectively. On the other hand, the treatment without application presented a root length of 5.8±0.16 cm and a root volume of 1.8±0.06 cm3 (Fig. 2A and B).
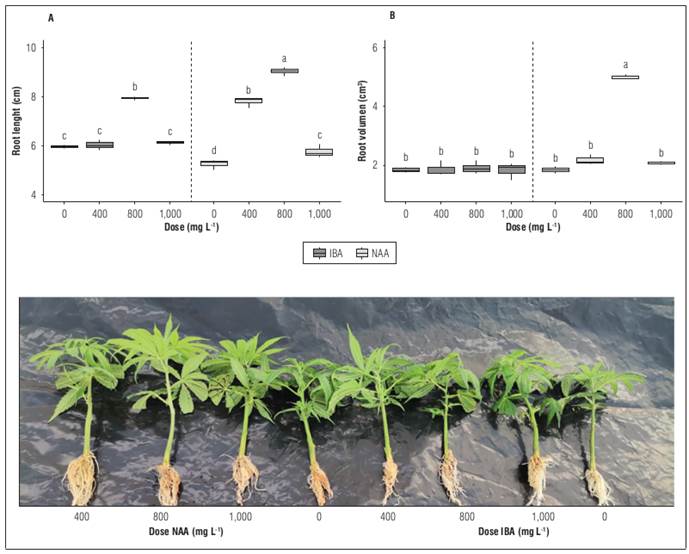
Figure 2. Behavior of hemp cuttings treated with different types of auxins phytoregulators applied in different doses: A, root length; B, root volume. Means with different letters indicate significant differences according to Tukey's test (P<0.05).
The application of auxin phytoregulators in solution was effective in inducing root development. Blythe et al. (2007) indicate that rapid immersion has several advantages, including uniformity of application, reduction in cost, and uniform rooting results. Cuttings with application of IBA presented changes in the length of the roots but not in their volume, while cuttings treated with NAA presented changes both in length and volume of roots (Fig. 2A, B). Media supplemented with NAA developed thicker roots, but this effect was suppressed as the concentration increased, in contrast to media supplemented with IBA in which all measurements increased as the levels of treatment increased, except for the quality of the explant.
A low response to IBA was observed in relation to rooting, but a positive response with the application of NAA. Stephen et al. (2023) indicate that the application of NAA in different doses generated considerable formation of calluses which increased as the concentration of auxin increased. The application of a dose of 1,000 mg L-1 generated a lower length and volume of roots. Kamga et al. (2018) mention that an excessive concentration of auxin negatively affected the percentage of rooted cuttings, slowing down the activity of stimulating rhizogenesis (regeneration of adventitious roots).
Rooting and sprouting
There was an increase in both the percentage of rooting of the cuttings as well as in the number of new shoots, with significant differences between treatments (P≤0.05). The application of NAA in a dose of 800 mg L-1 presented the highest percentage of rooting, at 86.6±3.3%, and number of new shoots, at 6±0.2. Treatment without application presented values of 43.3±3.3% and 3.1±0.2 (Fig. 3A and B).
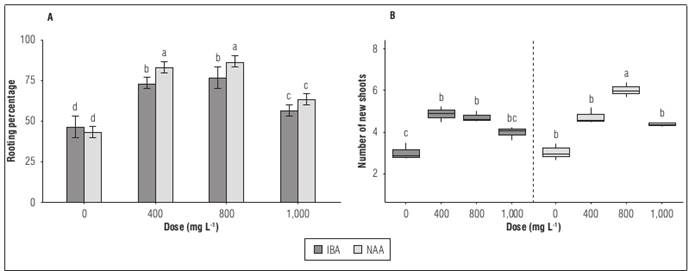
Figure 3. Behavior of hemp cuttings treated with different types of auxins phytoregulators applied in different doses: A. Rooting percentage; B. Number of new shoots. Means with different letters indicate significant differences according to Tukey's test (P<0.05).
Treatment with NAA at a dose of 800 mg L-1 generated an increase in the percentage of rooting of 100% compared to treatment without application and 10% compared to IBA treatment at a dose of 800 mg L-1 (Fig. 3A). Also, it generated a 100% increase in the number of new shoots, compared to treatment without application. Cuttings treated with NAA developed a significantly higher number of adventitious roots, with an increase in endogenous levels of IAA, which correlates with a decrease in the activity of AAOI examined (Chen et al., 2002).
The observed results indicate that the application of NAA as auxin phytoregulator to increase both the percentage of rooting and the number of new shoots improves the survival rate of the cutting. Saifuddin et al. (2013) indicate treatment with auxins increases the survival rate because of the rapid and adequate formation of roots in the cuttings. Similarly to what was observed in the previous variables presented, the application of a dose of 1,000 mg L-1 generated lower percentage of rooting and fewer shoots. Durango and Humanez (2017) indicate that as auxin concentrations increase, the number of roots decreases. Similarly, Sun et al. (2023) observed the same pattern as the NAA and IBA concentration gradually increased within the range of 0~200~500~800 mg L-1; the rooting rate, average number of roots, maximum root length and rooting efficiency index initially increased followed by a decrease when applying a concentration of 1,000 mg L-1. Salinas et al. (2022) indicate that the spatial pattern of auxin accumulation at the tissue level may be due to a combination of localized auxin biosynthesis and nonpolar (in areas of high auxin concentration) and polar transport through cells.
The observed positive effect of exogenous NAA is related to the alteration of the levels of other endogenous hormones, including ABA, which is generally considered an inhibitor of rooting of cuttings (da Costa et al., 2013). But according to Wang et al. (2022), when applying NAA, ABA levels were slightly increased, especially in green-stemmed cuttings, promoting rooting. Wang et al. (2022) indicate that the application of exogenous NAA significantly reduced the content of TZR (the most active cytokinin) in cuttings of the tea plant, promoting the formation of roots. Jing and Strader (2019) indicate that a high auxin: cytokinin ratio promotes adventitious root formation, while a relatively low ratio facilitates bud formation.
CONCLUSION
The type of rooting hormone strongly influenced the success and quality of adventitious rooting in hemp cuttings. This study found a positive response to the application of the auxin phytoregulator of NAA at a dose of 800 mg L-1. A lower response was also observed when applying 1,000 mg L-1 regardless of the type of auxin phytoregulator used. The results of this study can lead to a technical tool to improve the propagation process through cuttings of hemp materials grown in producing areas of Boyaca and the Colombia, provided that the different factors immersed in this process are validated.















