Markers in organ failure
Most organ failures are characterized by different serum markers very well known to the medical literature. Heart failure increases brain natriuretic peptide, renal failure increases serum creatinine levels, liver failure increases liver enzymes and so forth. These markers not only indicate organ insufficiency severity but also provide an indicator to organ function improvement. Now what about the gastrointestinal tract? Until recently, intestinal failure severity and improvement were matters of clinical and symptomatic observation to determine intestinal rehabilitation and the optimal moment of initiating oral feeding. During the last few years, biomarkers possibly proving germane to the digestive system have emerged. Here we discuss aspects of the present and future of intestinal failure biomarkers in short bowel syndrome.
Intestinal failure
In 1981 Fleming and Remington coined the term ¨Intestinal Failure¨ following the observation of the gastrointestinal tract unable to follow through with its function to absorb the adequate amount of nutrients necessary to sustain life. Intestinal failure is defined as the inability of the gastrointestinal system to absorb more than 81% of the basal metabolism 1,2,3. Classically intestinal failure is divided in three types, Type 1 which is a self-limiting, short-term (14 days) event secondary to post-op ileus, small bowel obstruction and gastroenteritis. This first type can be managed with total parenteral nutrition and nothing by mouth. The second type involves a complex, mid-term, delayed (15 days to 6 months) insult to the gastrointestinal tract rendering the patient dependent on total parenteral nutrition and possibly nutrition through enteric fistulas (fistuloclysis). This condition results secondary to high output stomas, entero-atmospheric fistulas and short bowel syndrome primarily; they eventually require reconstructive gastrointestinal surgery. Type 3 is a long-term intestinal failure (more than 6 months) secondary to extended and/or multiple intestinal resections resulting in a chronic inability of the digestive system to recover its function. It can be permanent and treatment options include bowel lengthening surgery and intestinal transplant. Patients often remain permanently on total parenteral nutrition if an adequate adaptation is not achieved 1,2,3.
Short bowel syndrome
In general, we offer the term “short bowel syndrome” in the context of a patient with less than 200 cm of small intestine. This decrease in anatomy and thus intestinal absorptive capacity results in intestinal failure. Taking intestinal resection anatomy into account, three anatomical and anastomotic types of short bowel syndrome can be described: a jejunostomy with less than 115 cm of small intestine, a jejunocolonostomy with less than 60 cm of residual small intestine without the ileocecal valve, and an ileojejunostomy with less than 35 cm of residual intestine conserving the ileocecal valve and colon 4,5,6. Since most nutrients are absorbed within the first 100-150 cm of the jejunum, and glutamine, the primary amino acid necessary in normal human metabolism, which is absorbed in the distal jejunum, significant malnutrition can result from short bowel syndrome. Adding glutamine at 0.3g/kg/d significantly reduces mortality in patients on total parenteral nutrition with short bowel syndrome. Cianocobalamine, bile acids and magnesium are absorbed primarily in the distal 100 cm of the ileum, with the proximal colon also contributing to magnesium absorption. Whenever the colon is present even in short bowel syndrome, unabsorbed carbohydrates pass into the colonic lumen and are fermented into short chain fatty acids and provide caloric value. Approximately 150-1000 kcal can come from this pathway 5,6,7,8.
Intestinal failure secondary to short bowel syndrome results in a devastating consequence of emergency and damage control surgery, rendering patients dependent on various aspects of nutritional technologies in order to recovery and lead a relatively normal life. As new research surfaces strategies in intestinal rehabilitation, the question remains, when is the optimal moment to re-initiate oral feeding and wean patients off of parenteral nutrition. The answer to this question may not be easily found, however biomarkers for intestinal failure may help approximate answers to this dilemma and clinical challenge, and result in a new era of intestinal rehabilitation 3,4,5,6,7,8.
Intestinal Rehabilitation
Intestinal rehabilitation is a physiological process of the remaining small bowel which involves recovery of its absorptive capacity through intraluminal stimuli by nutrients and gastrointestinal secretions. This process involves three phases, an acute phase of up to four weeks following major surgery in which gastrin regulation is lost and gastrointestinal loss is at its maximum. Primary complications during this phases includes dehydration, electrolyte imbalances and renal failure 2,9,10,11. The second phase can take up to two years, this stage involves an anatomical and histological regenerative process in which the intestinal lumen tries to optimize absorptive capacity through villi elongation and crypt deepening. Another important adaptation of the small intestinal is the slowing down of intestinal transit allowing more time for nutrient-mucosal contact and thus absorption. The last stage is determined by the degree of intestinal functionality during the second phase. The presence of the large intestine is important in this phase since it allows the maintenance of fluid balance, electrolyte stability and may determine parenteral nutrition weaning and whether the patient will be able to tolerate oral feeding permanently, remain on artificial nutrition or require intestinal transplantation 8,9,10,11,12,13.
From the moment that a patient is suspected of having or will shortly be ailing from intestinal failure, early parenteral nutrition is crucial. Electrolyte replenishing and an adequate urine output of 800-1000 cc is vital. During intestinal rehabilitation, cholestasis, hepatic failure, and central catheter infections are common consequences of parenteral nutrition that should be taken into account. Once patients recover form postoperative ileus and distension, which may take up to two to four weeks, oral feeding may be initiated taken into consideration that patients will persist with multiple episodes of daily diarrhea. Oral feeding may include isotonic solutions rich in sodium and other electrolytes; simple sugars should be avoiding at this point. Enteral nutritional supplementation cannot be recommended at this point since there is no evidence of its benefits in the literature 2,8,9,10,11,12. Once an adequate oral feeding has been established with proper tolerance, anti-diarrheal therapy should be implemented and parenteral nutrition may be cycled. Other elements in the medical management at this point include gastric protection, bile acid sequesters, and somatostatin analogs 9,10,11,12,13. When patients start approaching the second phase of intestinal adaptation, and have tolerated oral feeding along with a moderate diarrheal output, the following step includes the use of hyperplasic treatments such as growth hormone and Teglutide (GLP-2 analog produced by the terminal ileum and proximal colon). These medications reduce intestinal motility, increase splanchnic circulation and stimulate mucosal hyperplasia. 8,9,10,11,12 Once diarrheal output has been controlled and the patient has maintained adequate oral feeding sufficient to guarantee metabolic demands, parenteral nutrition weaning can be proposed and if successful, the rehabilitation process can be considered finalizing 3,10,11,12,13. Not every patient responds the same way during this entire process, to help determine and predict success rates, biomarkers may be implemented in patient management protocols.
Biomarkers in Short Bowel syndrome and Intestinal Failure
Over the years many biomarkers for intestinal failure have been proposed. These include cytokines, C-reactive protein, breath tests, I-FABP, I-BABP, DNA testing, fecal sampling, calprotectin, diamine oxidase, Citrulline, and until recently Apo-protein IVA (APO IVA) 14,15. Among these, the only two which have shown certain promising results in the prognostic and rehabilitative value in the management of intestinal failure in short bowel syndrome are Citrulline and APO IVA 14,15. Let us shortly review these two. Citrulline is an amino acid not incorporated into protein and is produced mainly by intestinal enterocytes from ornithine and glutamine and its metabolism finalizes in the kidneys by releasing arginine. Citrulline is not found in food with the exception of watermelon at 1g per 780g of fruit. Normal serum value is 40 mcmol/L and according to the severity of intestinal dysfunction this value may reduce. In intestinal failure and short bowel syndrome with a significant mass reduction in the intestinal anatomy, levels can decrease to 20 mcmol/L and in villous atrophic intestinal disease levels reach 10 mcmol/L 14,15,16. When serum levels reach 20 mcmol/L in the context of short bowel syndrome, permanent intestinal failure is predicted with a sensitivity of 92% and specificity of 90% 15,16,17. In prolonged starvation, serum levels can also drop by 30%. Two primary factors which may cause false negatives in patients with short bowel syndrome are age and renal failure; in both of these conditions serum levels may rise confusing the clinician. Between 140 cm and 160 cm of small intestine, serum Citrulline levels average 30-40 mcmol/L, whereas remnant lengths of 20-40 cm average 10-20 mcmol/L 14,15,16,17.
The second biomarker possibly germane to intestinal rehabilitation is APO IVA. APO IVA is a protein synthesized exclusively by enterocytes, primarily those in the ileum and accounts for 4% of proteins produced by these cells. APO IVA travels on chylomicron membranes and is released into plasma providing a normal serum level of 4.6 mg/100ml or 32 AU. Using a Western blot technique, APO IVA levels can be compared between healthy patients and those with short bowel syndrome 14,15,16,17. A cut-off value 4.6 mg/100ml gives a predictive value of intestinal rehabilitation and sets a score between patients who attain oral feeding vs those who remain on parenteral nutrition. Patients with approximately 150 cm of small intestine average serum APO IVA levels between 30 to 50 AU and those with small bowel remnants of less than 50 cm average serum APO IVA levels of 10 AU 14,15,16,17.
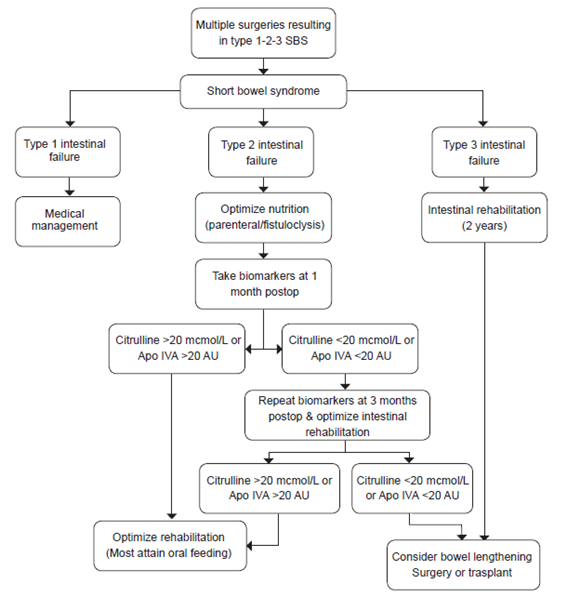
Biomarker levels are also affected by the type of anastomosis or anatomical intestinal remnant. This makes sense since the two primary biomarkers described here are produced by different areas of the small intestine, thus Citrulline levels are affected more by type I anastomoses and APO IVA levels by type II. The relationship between Citrulline and APO IVA values also reflect proportionally to the length of small intestinal, as intestinal remnant length is reduced, so do these markers. In patients without ileum however, these biomarkers are less specific since jejunal adaptation allows for distant production of these biomarkers, the same is true for major jejunal resections with accompanying ileum remnants 16,17. Figure 1 shows an algorithm that may help guide clinicians.
Conclusions
Although initial comparisons may vary, these studies may indicate that biomarkers offer predictive values in the intestinal rehabilitation process and success. Biomarker serum levels should be ordered within weeks following intestinal resections and follow-up during months thereafter. We recommend that these biomarkers should be ordered at the beginning of the intestinal adaptation process when oral feeding is begun and at 6 months post-operative to provide a significant comparison and predictive value. The literature lacks information on long term variations in the serum levels of Citrulline and APO IVA after parenteral nutrition weaning has been achieved and intestinal adaptation has been attained in short bowel syndrome.
Intestinal failure secondary to short bowel syndrome is a big problem, although not a hot topic. Those affected are high risk patients with significant morbidity rates and reintegration into daily routine continuous to be a challenge for the general surgeon. Intestinal biomarkers may provide a guide to establishing criteria in the management of these patients. Whether to establish the appropriate time of initiating oral feeding, predicting long term consequences of short bowel syndrome or determining prognostic success rates of rehabilitation. Determining which patients will recovery adequately and which do not still remain a clinical dilemma, however applying the use of intestinal biomarkers into patient care protocols may help guide the clinician in optimizing treatment and results possibly creating a new era in intestinal rehabilitation. Additional research is warranted to define the future of intestinal failure, adaptation, and the place of biomarkers during this process.