Introduction
A hot thyroid nodule is defined as an image that concentrates large amounts of a radiotracer compared to other nodules or the rest of the thyroid parenchyma, and is seen as a “hot spot” during the gamma study of a patient with hyperthyroidism1. Coexistence between thyroid cancer and hyperthyroidism is rare, as most of the hot nodules in patients with hyperthyroidism are considered benign; however, there is evidence suggesting an increasing number of documented cases of thyroid cancer in patients with toxic nodular goiter, toxic multinodular goiter, and toxic diffuse goiter, with no certain prevalence known at this time2. Most of these lesions are described as non-functional nodules adjacent to hot nodules or immersed in glands with diffuse tracer uptake, for example, cold nodules. However, there are some reports in the literature that inform the development of cancer from hot nodules in patients with hyperthyroidism, which forces us to preoperatively rule out the presence of cancer3. Unfortunately, there are no reports in the national literature that describe the development of cancer from hot thyroid nodules. In addition to documenting a relevant clinical case, the objective of this systematic review is to determine the possible clinical characteristics associated with the finding of thyroid cancer originating from hot nodules in patients with hyperthyroidism.
Case presentation
A 38-year-old female patient consulted for symptoms of at least 6 months of evolution consisting of palpitations, distal tremor in upper limbs and unconscious weight loss. On physical examination, a left lobe-dependent grade I goiter was documented, associated with 15 points on the Burch-Wartofsky Scale, consistent with a low probability of developing a thyroid storm. She presented with serum TSH levels demonstrating suppression and an ultrasound that reported a thyroid gland of heterogeneous ecogenicity and asymmetric lobar volumes, due to a hypoecogenic, partially defined borderline nodular image measuring 15 mm x 15 mm in relation to a thyroid nodule located in the left lobe, compatible with TIRADS category 4. No medical therapy was started because the patient referred that during the initial assessment done by endocrinology, she begun treatment with central and peripheral thyroid blockade, and presented allergic reaction to this medication. She is referred to the Endocrine Surgery Service of the E.S.E. Hospital Universitario del Caribe due to the impossibility of carrying out medical treatment.
After our assessment, a clinical impression of a toxic nodular goiter was made and a complete thyroid profile is requested, including antithyroid antibodies, thyroid scan and fine needle biopsy under ultrasound guidance of the nodular lesion, to define the etiological diagnosis of hyperthyroidism and indication of surgical management. Thyroid profile results were compatible with clinical hyperthyroidism, with levels of TSH 0.004 uUI/ml, total T4 of 12 ng/ml, free T4 of 1.67 ng/dl, free T3 of 3.98 pb/ml and total T3 of 1.1 pb/ml, presenting antibodies anti peroxidase thyroid 10 mg/dl and antithyroglobulin 20 mg/dl. The results of the thyroid scan showed an increased uptake in the middle third of the left thyroid lobe, which suppressed the rest of the parenchyma, with a capture index of 8.1 (Figure 1).
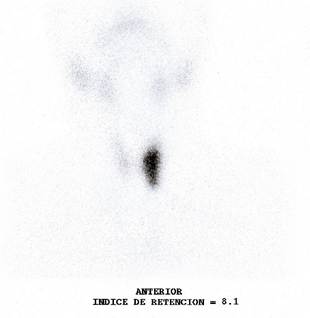
Image 1. Tc99 thyroid scintigraphy showing a hot nodule located in the left thyroid lobe, which suppresses tracer uptake in the rest of the thyroid parenchyma.
Evaluation of the cytological smears obtained by fine needle biopsy showed findings conclusive with malignancy, category VI of the Bethesda System (Figure 2). This result documented the diagnosis of a toxic nodular goiter, secondary to Cope’s disease with an uncertain behaving tumor of the thyroid gland. In view of a cytology suggestive of malignancy, a contrasted neck scan is requested and it documented images with heterogeneous capture of contrast material in pre-tracheal station and left paratracheal station of 10 mm and 15 mm, respectively, without evidence of suggestive images of adenomegaly in lateral stations.
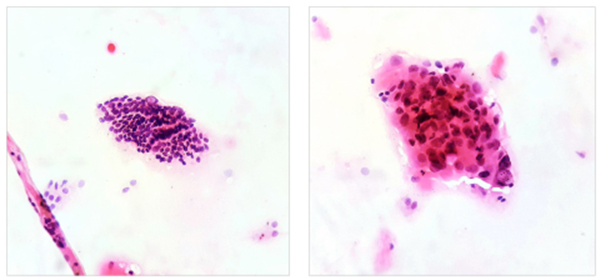
Image 2. Left,10x magnification. Groups of follicular cells with nuclear crowding are recognized, forming macrofollicles and microfollicles, some of the cells show intranuclear inclusions; Right, 40x magnification. A group of follicular cells with the presence of clefts, intranuclear inclusions, anisonucleosis and hyperchromasia is recognized.
Therefore, the patient is encouraged to have a total thyroidectomy with central neck lymphadenectomy. The macroscopic study of the surgical specimen showed a thyroid gland weighing 10 grams, in which a single nodule was observed, located in the middle third of the left lobe, pearly white in color and with a spiculated and irregular contour, which did not appear to be encapsulated; the rest of the parenchyma was of usual appearance (Figure 3). The microscopic study of the surgical specimen was compatible with an infiltrating non-encapsulated papillary carcinoma of follicular variant, associated with low-volume lymph node metastasis, represented by 2 of 8 nodes involved by the tumor lesion (Figure 4). The definitive diagnosis was a toxic nodular goiter secondary to a malignant tumor of the thyroid gland.
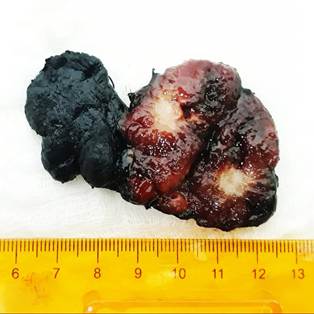
Image 3. Surface stained with India ink. A surgical specimen corresponding to the product of a total thyroidectomy weighing 10 grams is recognized. In the longitudinal section of the left lobe is recognized a poorly defined nodule, firm on palpation, pearly white in color and irregular contour with a starry appearance. Rest of the parenchyma of usual appearance.
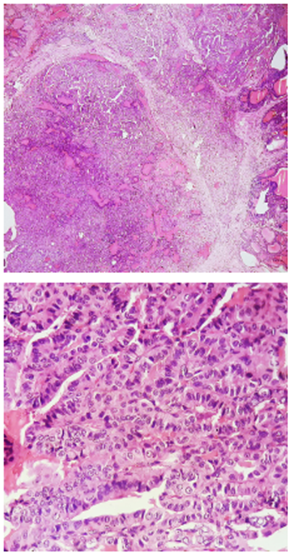
Image 4. Upper,10x magnification. Lower, 40x magnification. The sections show a malignant epithelial lesion, made up mostly of papillary structures and a minority of moderately defined follicular structures, lined by cuboidal to columnar cells with the presence of round to oval nuclei, with the presence of nuclear clearance, nuclear inclusions and fissures, adopting an infiltrative pattern.
Systematic review methodology
The systematic review process was standarized according to the parameters established in The PRISMA statement 4 and the systematization was carried out with the implementation of the Software Review Manager 5.3.
Articles published in English were searched in the Excerpta Medica database - Embase and Medline from January 1, 2001 to June 30, 2020, using the following terms: “thyroid cancer” OR “thyroid carcinoma” OR “papillary thyroid carcinoma” OR “papillary carcinoma” AND “hyperthyroidism” OR “thyrotoxicosis”.
A study was considered as one that addressed the core topic when in its title or in its structured abstract the relationship between thyroid cancer and hyperthyroidism was implicit. The inclusion criteria were: 1. The subject of study had to be defined as a surgical specimen; 2. The patient to whom the surgical specimen corresponds has been diagnosed with hyperthyroidism prior to the surgical procedure; 3. The diagnosis of hyperthyroidism was confirmed biochemically and categorized by thyroid scan; 4. The diagnosis of thyroid cancer was confirmed in the surgical specimen; 5. The lesion described as cancer on the surgical specimen corresponds to the hot nodule on the thyroid scan. The exclusion criteria were: CE00: Duplicate documents in databases; CE01: Article that does not address the topic and was conducted in a pediatric population; CE02: Systematic review that does not address the topic; CE03: Meta-analysis study that does not address the topic; CE04: Clinical case that does not address the topic; CE05: Original study that does not address the topic; CE06: Literary or systematic review that addresses the topic; CE07: Meta-analysis study addressing the topic.
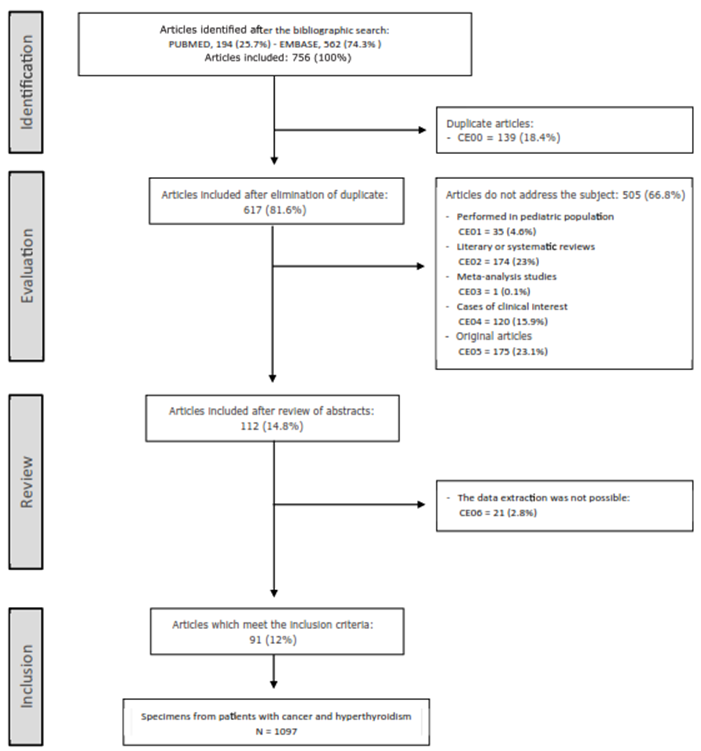
The application of the inclusion and exclusion criteria during the systematic review process is summarized in figure 5.
The definition of the variables and data for analysis were extracted from the methodology and results of the articles included in the systematic review. The scintigraphic features of the nodules from which cancer-compatible findings were documented were operationalized as a qualitative, nominal and dichotomous variable; it was categorized into two groups, hot nodule and cold nodule. The type of hyperthyroidism was defined with scintigraphy and was operationalized as a qualitative, nominal and polytomous variable. It was categorized into three groups: toxic nodular goiter, presence of a hot nodule that suppresses radiotracer uptake in the rest of the gland; toxic multinodular goiter, presence of one or more hot nodules that do not suppress radiotracer uptake in the rest of the gland; diffuse toxic goiter, presence of diffuse uptake in the gland. In this case the presence of uptake areas greater than the parenchyma uptake were considered as hot nodules, and areas with no uptake were considered as cold nodules. After a bivariate analysis for significance, contrasting the type of hyperthyroidism and the presence or absence of findings compatible with cancer documented from a hot nodule, the variable was dichotomized and categorized as follows: first group, compatible or not compatible with toxic nodular goiter, and second group, compatible or not compatible with toxic diffuse goiter. The histological type was operationalized as a qualitative, ordinal and polytomous variable; it was categorized into five groups: papillary carcinoma, follicular carcinoma, oncocytic carcinoma, anaplastic carcinoma and medullary carcinoma. After a bivariate analysis for significance, contrasting the histological type and the presence or absence of findings compatible with cancer documented from a hot nodule, the variable was dichotomized and categorized into two groups: compatible or not compatible with follicular carcinoma. Tumor size was operationalized as a qualitative, nominal and dichotomous variable; it was categorized into two groups: size ≤ 10 mm and > 10 mm.
The data extraction process was performed independently, and they were tabulated into two pilot databases. Subsequently, a researcher unified the two databases into a definitive database, and in cases where there was a discrepancy, the data from the corresponding article was extracted and verified by the same author.
Statistical analysis
The qualitative variables were presented in the form of frequency tables. Prevalence ratio (PR) and odds ratio (OR) were calculated. The statistical significance of this association was calculated using the Pearson Chi-square test. A stratified analysis was performed, adjusting the odds ratio for the diagnosis of a carcinoma from a hot nodule in the group of patients with toxic nodular goiter and toxic diffuse goiter, depending on the histological type and tumor size. The homogeneity of the advantage ratio was calculated by means of the Mantel-Haenszel Chi-square test and the natural logarithm of the effect estimation was used. Effect independence was determined for each of the covariates using the Breslow & Day statistic. A binary logistic regression model was proposed to predict the probability of making the diagnosis of cancer from a hot nodule based on the type of hyperthyroidism, histological type and tumor size. The variance of the diagnosis of hyperfunctioning carcinoma was evaluated with Negelkerke’s R2 model and its statistical significance was assessed using the Hosmer & Lemeshow statistics. P values less than 0.05 were considered statistically significant. Data analysis was performed with the statistical package IBM SPSS ® Statistics 25.0.
Results
Seven-hundred-fifty-six potentially eligible papers were identified. After applying the exclusion criteria, a total of 91 papers were included5-95. The sequence of inclusion and exclusion of articles is summarized in figure 5.
Inferential Bivariate Analysis
The prevalence of cancer in hot nodules was statistically higher in specimens with toxic nodular goiter (PR 18.22; 95% CI 1,567 - 2,119). The probability of identifying cancer in hot nodules in a specimen with toxic nodular goiter was statistically higher (OR 31.00; 95% CI 18.69 - 51.44). This relationship was statistically significant with a p value < 0.001. The prevalence of cancer in hot nodules was statistically lower in specimens with diffuse toxic goiter (PR 0.757; 95% CI 0.711 - 0.805). The probability of identifying cancer in hot nodules in a specimen with diffuse toxic goiter was statistically lower (OR 0.036; 95% CI 0.018 - 0.072). This relationship was statistically significant with a p value < 0.001. The prevalence of cancer in hot nodules was statistically higher in specimens corresponding to follicular carcinoma (PR 1,592; 95% CI 1,330-1,906). The probability of identifying cancer in hot nodules in a specimen corresponding to follicular carcinoma was statistically higher (OR 10.84; 95% CI 6.521 - 18.02). The prevalence of cancer in hot nodules was statistically higher for tumor lesions > 10 mm in size (PR 7.595; 95% CI 4.097 - 14.08). The probability of identifying cancer in hot nodules in a tumor lesion with size > 10 mm was statistically higher (OR 8.771; 95% CI 4.629 - 16.67).
Stratified Inferential Analysis
The diagnosis compatible with toxic nodular goiter increased the probability of making the diagnosis of cancer in hot nodules independently of the presence or absence of follicular carcinoma as histological type. The increase in the probabili- ty of making the diagnosis of hot nodule cancer in the group of patients with and without toxic nodular goiter was heterogeneous depending on the presence or absence of follicular carcinoma as a histological type, and the magnitude of the increase was greater in the group of specimens with follicular carcinoma. The probability of making the diagnosis of cancer in hot nodules was higher in specimens with toxic nodular goiter and follicular carcinoma (OR 3.255; 95% CI 2.763 - 3.747). This association was statistically significant with a p value < 0.001. The diagnosis compatible with toxic nodular goiter increased the probability of making the diagnosis of cancer in hot nodules independently of the presence or absence of a tumor lesion > 10 mm. The increase in the probability of making the diagnosis of cancer in hot nodules in the group of patients with and without toxic nodular goiter was heterogeneous according to the presence or absence of a tumor lesion > 10 mm, and the extent of the increase was greater in the group of specimens with tumor lesions > 10 mm. The probability of making the diagnosis of cancer in hot nodules was higher in specimens with toxic nodular goiter and tumor lesions > 10 mm (OR 3.343; 95% CI 2.821 - 3.866). This association was statistically significant with a p value < 0.001.
The diagnosis compatible with diffuse toxic goiter decreased the probability of making the diagnosis of cancer in hot nodules independently of the presence or absence of a tumor lesion > 10 mm. The increase in the probability of making the diagnosis of cancer in hot nodules in the group of patients with and without diffuse toxic goiter was heterogeneous according to the presence or absence of a tumor lesion > 10 mm, the extent of the increase was greater in the group of specimens with tumor lesions > 10 mm. The probability of making the diagnosis of cancer in hot nodules was higher in specimens with diffuse toxic goiter and tumor lesions > 10 mm (OR 0.306, 95% CI 0.253 - 0.387). This association was statistically significant with a p value < 0.001.
Multivariate Inferential Analysis and Binary Logistic Regression Model
A surgical specimen corresponding to a toxic nodular goiter (OR 6,406; 95% CI 3,368 - 12,19), a tumor size > 10 mm (OR 5,524; 95% CI 2,717 - 11,12), and a histological type compatible with follicular carcinoma (OR 4,678; 95% CI 2,341 - 9. 344), independently increased the probability of making the diagnosis of a hot nodule cancer, while a surgical specimen corresponding to a diffuse toxic goiter (OR 0.120; 95% CI 0.051 - 0.281), independently decreased the probability of making the diagnosis of a hot nodule cancer. This relationship was statistically significant with a p value < 0.001. The relationship between the type of hyperthyroidism, histologic type and tumor size, with the prevalence of cancer in hot nodules is summarized in table 1.
Table 1. Relationship between the type of hyperthyroidism, histologic type, and nodular size, with the prevalence of thyroid cancer from hot nodules.
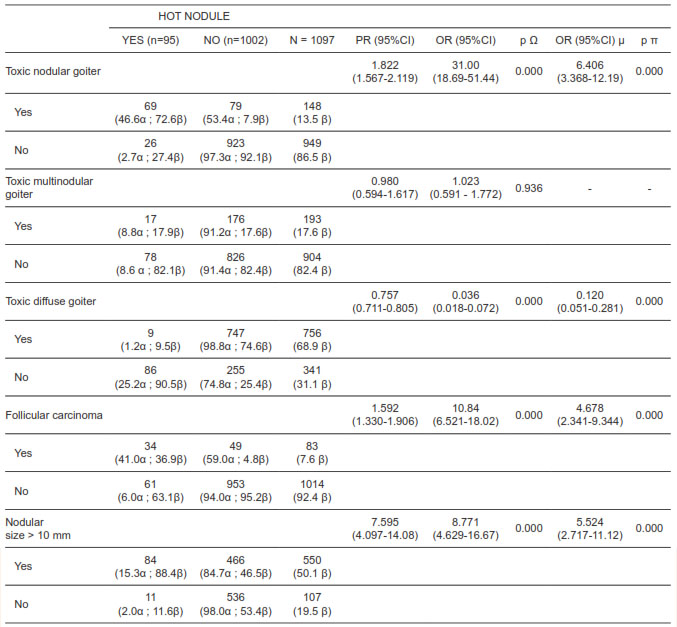
(a) Prevalence as a function of the presence or absence of the independent variable. (ß) Prevalence as a function of the presence or absence of the dependent variable. (µ) OR adjusted according to multivariate inferential analysis of binary logistic regression. (O) Pearson’s Chi-Square statistic. (p) Wald statistic.
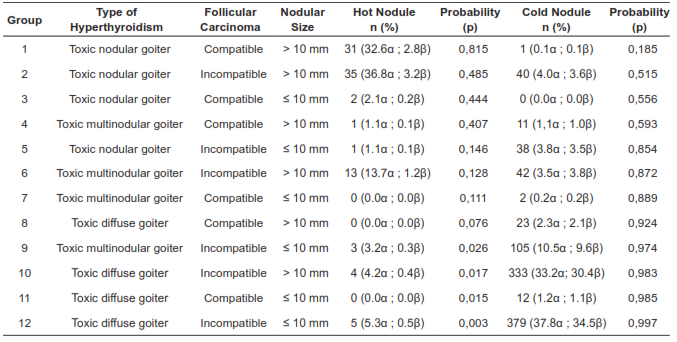
The binary logistic regression model built with the independent variables is summarized in table 2. It explains 52% of the variance in hot nodule cancer diagnosis and correctly classifies 94.1% of the cases. According to the Hosmer-Lemeshow statistics, this model was statistically significant with a p value = 0.010. According to this, the probability that a hyperfunctioning nodule corresponds to a malignant tumor, depending on the presence or absence of the variables included in the model, corresponds to the following equation: P = 1 / (1 + e - (LOGIT) where LOGIT = -1.918 + (1.857 x toxic nodular goiter) + (-2.119 x toxic diffuse goiter) + (1.543 x follicular carcinoma) + (1.707 x diameter > 10 mm).
Table 2. Probability of diagnosing a malignant tumor from a hot nodule according to the binary logistic regression model, P = 1 / (1 + e - (LOGIT)), where LOGIT = -1,918 + (1,857 x toxic nodular goiter) + (-2,119 x toxic diffuse goiter) + (1,543 x follicular carcinoma) + (1,707 x diameter> 10 mm). a) Frequency according to the group of cold nodules and hot nodules. (ß) Frequency as a function of the study population.
Discussion
Our revision confirms that in surgical specimens of patients diagnosed with hyperthyroidism and thyroid cancer, most of the malignant tumors derive from a cold nodule. However, 8.6% of the cases of cancer identified in the present review derived from a hot nodule, which forces us as clinicians to preoperatively rule out the presence of cancer in this group of patients. The proposed binary regression model suggested that clinical characteristics such as a clinical diagnosis corresponding to toxic nodular goiter, a histology compatible with follicular carcinoma, and a nodular diameter > 10 mm, on their own, are clinical characteristics capable of increasing the probability that a hot nodule corresponds to a malignant tumor, while a clinical diagnosis corresponding to diffuse toxic goiter decreases this probability.
Regarding the histological subtype, the results suggest that the prevalence of follicular carcinoma increases up to 41% (OR 4.678; 95% CI 2.341 - 9. 344) when it is calculated from the group corresponding to hot nodules. However, is important the fact that 63% of the hot nodules, almost two thirds of this group, corresponded to a papillary carcinoma, reason for which a slight increase in the finding of follicular carcinoma in hot nodules is suggested, compared to the usual presentation of the same in nodules in general, therefore this characteristic could be proposed as a possible risk factor between both variables in future studies.
In order to give clinical applicability to this model, it is important to clarify the possibility of documenting these characteristics preoperatively. Thus, the thyroid scan would allow the categorization of patients according to the probable etiology of hyperthyroidism, in addition to documenting the distribution of hot and cold nodules; the thyroid ultrasound would allow the determination of the diameter of the nodular lesions and categorize them as ≤ 10 mm and > 10 mm. However, it is not possible to determine preoperatively that the histological type corresponds to a follicular carcinoma, because the cytology obtained by fine needle puncture is not capable of preoperatively diagnosing this tumor lesion96,97. As a clinical alternative there is the option of categorizing patients according to the presence or absence of nuclear changes compatible with follicular neoplasia in the cytological smear.
As described in table 2, it is possible to suggest that patients with the factors corresponding to group 1 have the highest probability that a hot nodule corresponds to a malignant tumor; similarly, patients with the factors corresponding to group 12 have the highest probability that a cold nodule corresponds to a malignant tumor. The probability attributable to the diagnosis of cancer from hot nodules for the rest of the group should be applied according to the clinical context of each patient, and in particular, in patients with characteristics corresponding to group 7, group 8, and group 11, the probabilities calculated from the regression model are not clinically applicable, which is justified in that no cases of cancer from hot nodules were documented in these groups.
The methodological attributes that support our binary logistic regression model are subject to selection biases, as the data used in the statistical analysis corresponded to a tertiary sources. Therefore, we invite the academic community to carry out prospective studies, in which it is verified that the prevalences and probabilities exposed in the present model correspond to the real clinical observations, where homogeneous measurement of the factors described can be performed, and propose other possible clinical characteristics that can be associated to the diagnosis of cancer in hot nodules.
Conclussion
The possibility of diagnosing a malignant tumor from a hot nodule must be taken into account in clinical practice. The clinical diagnosis corresponding to toxic nodular goiter, a histology compatible with a follicular carcinoma, and a nodular diameter > 10 mm, on their own, are clinical characteristics capable of increasing the probability that a hot nodule corresponds to a malignant tumor. Prospective studies should be conducted to evaluate the clinical characteristics described here, and to propose other possible factors associated with the diagnosis of cancer derived from hot nodules.