Introduction
The incidence of well-differentiated thyroid carcinoma has been increasing in recent decades 1,2. Patients with this type of tumor have a good long-term prognosis, and survival has been reported to be higher than 98% at 10 years 3. The cornerstone of treatment is surgical resection with radioiodine (RAI) ablation and hormonal suppression 4. Although this treatment is highly effective and survival is long, there are expected adverse effects in these patients. There is a notable risk of recurrence that requires long-term follow-up with laboratory tests and ultrasonography as well as a potential need for additional surgical procedures or immunotherapy. Additionally, most patients need lifelong hormonal support and continued surveillance of their TSH levels. All these factors decrease quality of life (QoL) due to their effects on psychological, functional and physical domains 5,6.
Due to the increasing incidence of thyroid cancer 1, the number of thyroid cancer survivors who suffer the late effects of treatment has also increased. Many studies have demonstrated that thyroid cancer patients have an impairment of QoL in terms of anxiety, chronic fatigue, mental concentration, and muscle cramps and pain 7-10. However, most QoL evaluations have been made with generic instruments, such as the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-H&N35) 11 and the Hospital Anxiety and Depression Scale (HADS) 12,13. Although the number of published studies about QoL in thyroid cancer patients is substantial, many have small sample sizes or use non-validated instruments.
In 2013, Husson et al. 4 developed the health- related quality of life questionnaire for thyroid cancer survivors (Thyca-QoL), a specific instrument to assess QoL in thyroid cancer patients, following the parameters defined to design this type of tools and was also psychometrically validated and applied in other countries. The number of studies about QoL in thyroid cancer in Latin-American Spanish-speaking populations is minimal, and few of them use a specific validated instrument 11,14-16. As quality of life varies between populations, socioeconomic and cultural environments, it is necessary to validate a specific instrument to obtain information about this outcome.
The aim of this study was to generate a cross-cultural adaptation of the Thyca-QoL in the Spanish language and to assess its psychometric properties in a Latin American population.
Methods
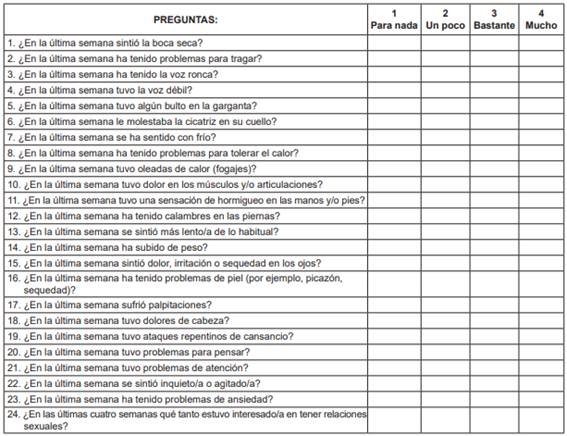
This study utilized a descriptive and prospective cross-sectional design to validate the Thyca-QoL instrument in patients with thyroid cancer. The author was contacted to receive authorization for use of the instrument, and the original version of the Thyca-QoL was obtained from Husson et al. 5. The Thyca-QoL has 24 questions, seven domains (neuromuscular, voice, concentration, sympathetic, throat/mouth, psychological, and sensory) and seven single items (scar problems, felt chilly, tingling hands/feet, gained weight, headaches, and interest in sex). Each item has four response categories (1 for ‘not at all’, 2 for ‘a little’, 3 for ‘quite a bit’, and 4 for ‘very much’) and specifies a time frame (one week for most items; four weeks for the sexuality items). A higher score represents a worse result. The questionnaire can be self- administered or completed with assistance.
We followed the Professional Society for Health Economics and Outcomes Research (ISPOR) guidelines for the translation and validation of health-related scales 17-19. The first translation was performed by two independent native English translators. A consensus document was back-translated by two independent native Spanish translators. The final document was compared with the original instrument and was evaluated by two English-fluent native Spanish surgeons to detect differences from the original scale. A pilot test was performed with ten patients to assess readability (Table 1). We asked candidates to complete the Spanish-validated versions of the Thyca-QoL and the EORTC QLQ-C30 under interviewer supervision. The inclusion criteria were as follows: adult patients with histologic confirmation of thyroid carcinoma who underwent total or partial thyroidectomy for whom Spanish was the native language. Patients with physical impairments that prevented reading, hearing or understanding the scale, and those who did not consent to participate were excluded. All patients were treated by the same surgical group. We also obtained demographic characteristics, comorbidities, ECOG scores, TNM stage (UICC 7th version), and data on the surgical procedure, adjuvant treatment with radioiodine, T4 supplementation, surgical complications and vital stage data from the clinical charts. For the test-retest analysis, randomly selected patients completed the Thyca-QoL instrument for a second time two weeks after the first evaluation.
Table 1. Spanish version of the thyroid-cancer-specific Thyca-QoL scale - Escala de calidad de vida THYCA-QOL para pacientes con cáncer de tiroides.
A sample size of 296 patients was calculated using the Bonnet formula 20 with the following parameters: a type I error of 0,05, a power of 80%, a Cronbach’s alpha estimate of 0,86, and a scale of 24 items. A test-retest analysis was planned in a random subsample of 45 patients.
The categorical variables are presented as percentages and ranges, and the continuous variables are shown as the mean and standard deviation. We set the significance level to p<0.05. We used Stata statistical software (StataCorp, TX, USA, Version 9.1) for the psychometric validation, and we used an exploratory principal axis factor analysis with oblimin rotation to identify the underlying dimensions as measures of construct validity and to provide evidence regarding whether the instrument reproduced the same factor-loading pattern observed for the original Thyca-QoL 5. The assumptions of the determinant of the correlation matrix, Bartlett’s test of sphericity for intercorrelation, and the Kaiser-Meyer-Olkin measure of sampling adequacy for factor analysis were used. Also, a confirmatory factor analysis was done using the instruction valid scale of Stata. A reliability test involving stratified Cronbach´s alpha for the global scale, for the domains and the item-total correlation was performed. Values higher than 0.70 were considered acceptable. For the convergent/concurrent validity, we selected the Spanish version of the EORTC QLQ-C30, and we used the type of surgical procedure for the known-group validity. The scores were compared using Pearson’s correlation coefficients. For the test-retest reliability, we used the intraclass correlation coefficient.
Results
Characteristics of the population
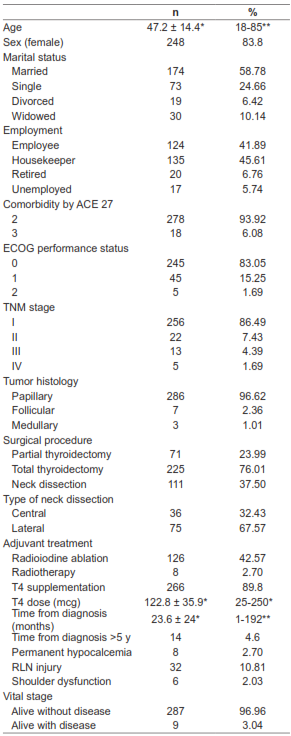
We interviewed 296 patients. Table 2 shows the characteristics of the patients. The mean age was 47.2 ± 14.4 years (median 46, range 14-84). Most patients were married females, and 45% were housekeepers. The most common tumor was papillary carcinoma, and 94% of the patients had TNM stage I/II disease. Only 6% had severe comorbidities. A total of 76% of patients underwent a total thyroidectomy and 37% needed a concomitant neck dissection. Adjuvant treatment was administered to 45% of patients, and 90% received T4 supplementation. The overall rate of postoperative complications was 14%.
Table 2. Demographic and clinical characteristics of the cohort of patients with thyroid cancer.
*Mean, ± sd, ** range, ACE 27: Adult Comorbidity Evaluation-27; ECOG: Eastern Cooperative Oncology Group; TNM: tumor, lymph nodes and metastasis.
Cross-cultural adaptation
The translations produced a draft with some adaptations:
1. The word “lump” in the item 5 was adapted to “mass” (Spanish term “bulto”), a more common term used in Latin-America.
2. The term “hot flushes” in item 9 was adapted to “fogajes” in Spanish (very common term used by women to refer to postmenopausal symptoms).
To evaluate readability a pretest was made in ten patients (two of them healthy 12-year-old children). No changes in words were made after this phase. A change in the format of the scale was suggested, with increasing the size of the letters and widening space. In general, there was good agreement between the translated and original English versions of the questionnaire. The final version of the Thyca-QoL scale can be found in Table 1.
Item description
The mean score for the Thyca-QoL scale was 42.3 ± 10.8 (median 40, range 25-86). The items with poorer quality (i.e., experienced ‘quite a bit’ and ‘very much’ responses) were joint/muscle pain, headache, tiredness, and fatigue. The items with better quality (i.e., ‘not at all’, ‘a little’ responses) were attention problems, palpitations, lump in the throat, problems with neck scar, and trouble swallowing. (Figure 1).
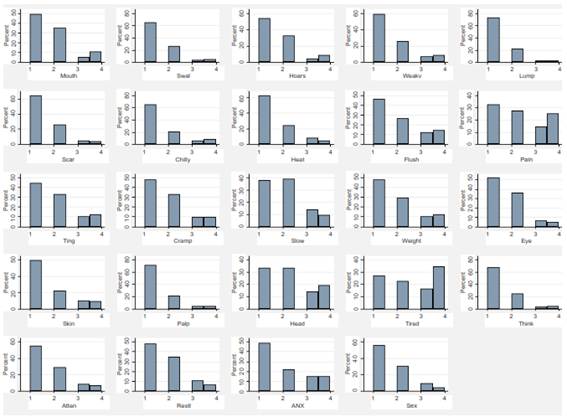
Figure 1. Distribution of the Thyca-QoL scores per item. *Figures and tables are original from the study data.
Construct validity
Factor analysis was performed by replicating the methods of the original study. Exploratory principal axis factor analysis showed a seven-factor solution using the Kaiser criterion (eigenvalue>1). The seven factors explained 58% of the variability (Table 3). The diagnostic tests were good (the Kaiser-Meyer-Olkin value was 0.84, and the Bartlett sphericity test yielded a p<0.001). The confirmatory factor analysis with the asymptotic distribution free method reported a p=0.000 for the chi-square test, a root mean square error of approximation (RMSEA)=0.053, a standardized root mean square residual (SRMR)=0.1, and confirmatory factor index (CFI)=0.86 which are indicators of moderate fit.
Table 3. Factor analysis of items included in the Thyca-QoL scale.
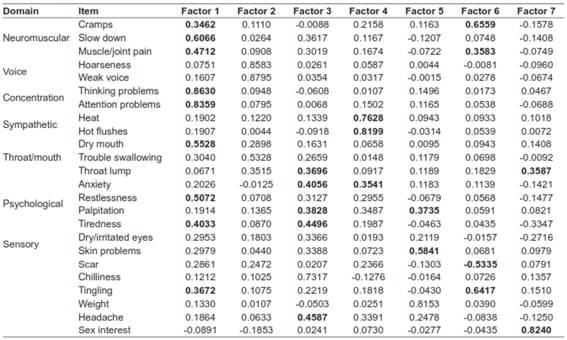
*Bold correspond to coefficient >0.35.
Internal consistency reliability
Stratified Cronbach’s alpha for the global scale was 0.92. The results for individual items to assess convergent and divergent validity are shown in Table 4. All Cronbach’s alpha values for individual items were >0.7. The throat lump, scar, chilliness, weight, and sex interest items had item-test correlations <0.4.
Table 4. Reliability, item-scale, and item-rest correlation for items and subscales of the Thyca-QoL scale.
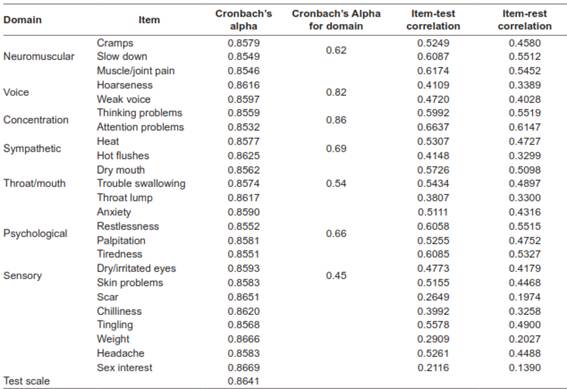
Concurrent validity
The comparison between the Thyca-QoL and the EORTC QLQ-C30 demonstrated a high correlation (rho= 0.75) (Table 5). For the known group validity, patients with ECOG score>0 showed a higher Thyca-QoL score compared with those having ECOG=0 scores (50.9 vs. 40.5; p<0.001), and patients who received adjuvant radiotherapy had lower scores compared with those who did not (36.2 vs 42.5; p=0.11). However, we could not find differences in Thyca-QoL scores according to TNM stage (I/II vs III/IV, 42.4 vs 41.4, respectively; p=0.72), comorbidity (ACE-27 2 vs ACE-27 3, 42.1 vs 46.1, respectively; p=0,13), adjuvant treatment use (with vs without, 43.4 vs 41.4, respectively; p=0.11), neck dissection (yes vs no, 42.6 vs 42.1, respectively; p=0.72), and complications (yes vs no, 43.3 vs 42.1; p=0,53). The voice domain showed lower scores in patients with recurrent laryngeal nerve (RLN) injury (3.1 vs 5.1; p<0.001), and the tingling domain score was lower in patients with permanent hypoparathyroidism (2.5 vs 1.8; p=0.09).
Table 5. Pearson’s correlation coefficient between the Thyca-QoL scale and the EORTC QLQ-C30.
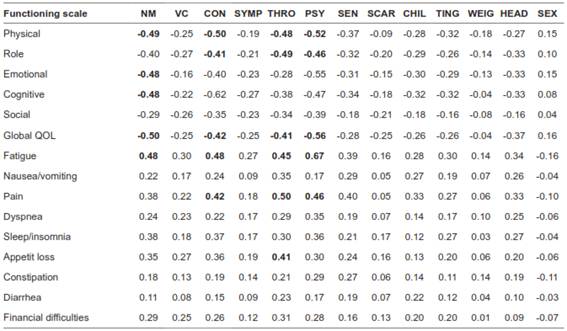
NM, neuromuscular; VC, voice; CON, concentration; SYMP, Sympathetic; THRO, throat/mouth; PSY, psychological; SEN, Sensory; CHIL, Chilliness; TIG, tingling; WEIG, weight; HEAD, headache; SEX, sexual. *Bold correspond to coefficient >0.40.
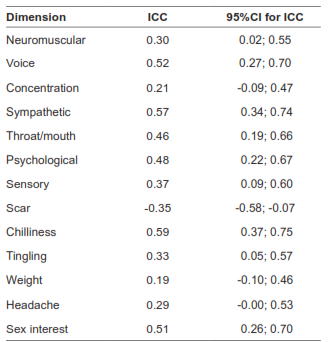
Test-retest reliability
In a sample of 45 patients, the concordant correlation coefficient for the global scale was 0.87. Table 6 shows the reproducibility results. The domains with higher values were voice, sympathetic, chilliness, and sex interest. The scar domain showed a negative coefficient, which is consistent with improvement in the quality of the scar with time.
Discussion
QoL assessment is an important method to determine the effects of disease and its treatment. For thyroid cancer, QoL assessment has major relevance because mortality and recurrence are low, and therefore, people live with the diagnosis for a longer time. Recent information suggests that QoL in thyroid cancer patients can be as low as that reported in other types of tumors with worse prognosis 21. Unfortunately, there are no specific validated Spanish-language instruments, and the few available studies use generic instruments. Novoa et al. 14 assessed QoL in 75 patients with thyroid cancer in Colombia using the SF-36 instrument and found low scores in the physical, social, and mental domains, and Vega-Vasquez et al. 16 evaluated 75 patients in Puerto Rico using the UW-QOL instrument and found low scores in the physical and social subscales. Four hundred million people speak Spanish in Latin America and Spain, and almost 40 million people speak Spanish in the USA, so it is expected that there is a large number of patients who could benefit from the use of a language-specific validated instrument.
The Thyca-QoL was designed by Husson et al. 5 in 2013 as a specific instrument to measure QoL in patients with thyroid cancer and was the first to follow standard methodological guidelines. It was developed from a sample of 306 patients at various clinical stages who had received a wide spectrum of treatments, and it showed good results in reliability. In 2015, Jeong et al. 22 validated the Thyca-QoL scale translation to the Korean language in a cohort of 227 patients with similar results to those reported originally. In this study, we made the validation of a cross-cultural adaptation of the Thyca-QoL scale. The process followed all the methodological steps necessary to guarantee the performance of the instrument. The measurements of the internal validity, reliability and reproducibility reached similar results as the original validation developed by Husson et al. 5 and the validation developed by Jeong et al. 22. The factor analysis showed a solution with seven factors that resembles the original results. However, the distribution of items by factor was different from those reported by Husson et al. 5. The voice (Factor 2), concentration (Factor 1), and sympathetic (Factor 4) domains maintained the same distribution, but the other domains had a different distribution. This can be explained by the differences in value given to each symptom by different populations. This study included only Latin American patients, where factors such as weight are commonly attributed to thyroid gland dysfunction and where scarring is very relevant in daily life. However, the Spanish version increased the percentage of variability explained by the instrument compared with the original version (58% vs 46%). The reliability of individual items was high, but the domain analyses showed a decrease in Cronbach’s alpha. However, our values are very similar to those originally reported, and the item-test coefficients were higher than 0.4.
Concurrent validity assessment showed a high Pearson’s coefficient for the global score. The comparison by domains with the EORTC QLQ-C30 showed a similar pattern from those originally described, but the number of items with Pearson correlation coefficient >0.4 was low. Reproducibility was high for voice, sympathetic, sex, and chilliness domains and moderate for the others. Finally, a known group evaluation showed that the instrument had the ability to discriminate between clinical conditions such as the ECOG functional scale and complications. We also improved some of the previous weaknesses of the Husson et al. 5 study, such as including only 5% of patients who were more than 5 years post diagnosis, to avoid a floor/ceiling effect. Additionally, we included a greater variety of treatments, such as radiotherapy, and more advanced tumors according to the TNM staging (22.5 vs 6%), which allows wider applicability. All these measures assure that the Spanish version of the instrument can be used.
This study has some weaknesses to report. Some of our patients were without T4 support during their preparation for RAI ablation, and this factor can affect the evaluation of QoL. The design was cross-sectional, so QoL was measured only once. As QoL is a continuum that can change with different treatments, this design impedes the evaluation of these changes. A social desirability scale was not administered parallel to the Thyca-QOL, and therefore its effect on psychometric measures is unknown.
Conclusion
The results of this study provide a reliable and objective instrument to be used in clinical practice and for research objectives. The instrument can be used to monitor the daily impact of treatment and to provide a confident tool to evaluate strategies to mitigate treatment effects, realizing that some domains as scar does not have stability on time. Additionally, it is an important tool to be included in clinical trials that evaluate novel alternatives for thyroid cancer. The recent use of immunotherapy as a treatment for radioiodine-resistant tumors has demonstrated an improvement in survival, but its impact on QoL has not been adequately evaluated.