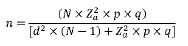INTRODUCTION
Bovine Leukemia Virus (BLV) is the agent of enzootic bovine leukosis (EBL), a neoplasm of lymphatic tissues in bovine species 1,2,3,4, BLV is classified into the genus Deltaretrovirus in the family Retroviriadae3. EBL is characterized by three disease stages: asymptomatic or aleukemic (AL), persistent lymphocytosis (PL), and leukemia or lymphoma 5. The majority of infected animals remain healthy without apparent negative economic effects, but BLV carriers develop a form of the disease known as persistent lymphocytosis (PL) in 30% of infected animals, while 0.1-10% of BLV-infected animals will develop either leukemia or lymphoid tumors 1,3,6,7.
During BLV infection, reduced cellular immune responses play major roles in disease progression and lead to increased susceptibility to other infections 6,7,8, BLV reduces anti-viral cytokine activities by suppression of interferon-gamma (IFN-g) and Tumor Necrosis Factor (TNF-a), and natural killer (NK) cytotoxicity by inducing transforming growth factor (TGF)-β secretion from regulatory T cells, suggesting that TGF-β from T cells type CD4+CD25highFoxp3+ is immunosuppressive and contribute to diseases progression 9.
Iatrogenic infection through blood-contaminated needles, surgical instruments, and gloves for rectal palpation is probably the major route of transmission in most settings 10,11. Field transmission of BLV requires direct prolonged contact between animals or exchange of blood, exudates, or tissues (e.g. during parturition), the spread of BLV between herds can occur via infected animals, while semen, or hematophagous insects probably account for an only small proportion of cases 10. Infection of BLV, according to detected by serology, is more typical in older cattle than the younger cattle 12.
EBL occurs worldwide in cattle-raising countries. In most of these countries, EBL is a notifiable disease, and official control measures include screening or monitoring, precautions at borders, control of movement inside the country, and stamping out 10. EBL has been eradicated in 22 countries worldwide through national control program, in many European countries in recent years their cattle population is relatively free from BLV 5,13,14.
In fact, for the Caquetá state, only a study about prevalence of EBL has been developed by 15 more than 15 years ago, and a new study for to determinate and to upgrade the prevalence of BLV is necessary taking into account that the bovine population in this state is amounts of 1,686,852 animals, and this state is the fifth dairy basin more important of Colombia, with a milk production higher to 1.215.833 kg per day16.
The aim of this research was determinate the prevalence of bovine leukemia virus in dual-purpose cattle over 36 months of age, in the nine municipalities that carrying the 75% of bovine population in the Caquetá state.
MATERIALS AND METHODS
Area of study. The research was performed in nine municipalities from Caquetá State, located to south of Colombia and the northeast of the Colombian Amazonia, between the 00°42’17” of latitude south and 02°04’13” of latitude north, and the 74°18’39” and 79°19’35” of longitude west of Greenwich. Borders to the North with both Meta and Guaviare states, to east with both Vaupes and Amazonas states, to south with the Putumayo and the west with both Cauca and Huila states; the Caquetá state have an extension of 88,965 km2 and is politically divided in 16 municipalities 17. The municipalities involved in the study were: Albania, San José del Fragua, La Montañita, Milán, El Paujil, El Doncello, Cartagena del Chairá, Puerto Rico and San Vicente del Caguán.
Caquetá state has three thermal floors (cold, temperate and warm). In warm climate that covers the greatest area to state, the average precipitation is of 3.800 mm/year, without dry station well defined, (nevertheless, the lower precipitation is registered between December to January and the greater precipitation between March to November), the rainfall erosivity index (R or EI30) multiannual is the 2.750 tm/cm/ha/h, value three times greater than other regions of country, the relative humidity is greater than 80%, but can fluctuate between 64 to 93%, with temperatures range between 18 to 36°C with average annual of 25°C characteristic of a regime isohyperthermic. The evapotranspiration potential is 1.435 L/m2/year, the average of solar radiation is of 1.800 hours/year and the intensity of 268 cal/m2/day 18,19,20. The climatic characteristics in the study area are of tropical rainforest according to Holdridge Life Zone Ecology 21.
Methodological design. A transversal study for estimate the sero-prevalence of bovine leukemia virus in dual-purpose cattle in the Caquetá state was performed. The probability of success was determinate in a level of 50%. A stratified random sampling of herds was made, taking into account herds dedicates to dual-purpose production, bovine females in milk production and bulls of each farm.
The sample size. Using the information of the agricultural assessment 2016 of the secretary of Agriculture of Caquetá state, the total of bovines females over 36 months of age were of 374 317. Was calculated the sample size from a known population, using the following equation:
Where n = sample size, N = population size (374,317), Z = confidence level (p<0.05), p=probability of success (0.5), q = probability of failure (0.5), and d = precision (maximum allowable in terms of ratio error) (3.5%). So, the sample size was estimated at 782 females over 36 months of age.
For estimation of sample size of dual-purpose herds the previous equation was used with the following assumptions: N = population size (11.128), Z = confidence level (p<0.05), p = probability of success (0.5), q = probability of failure (0.5), and d = precision (maximum allowable in terms of ratio error) (10%), thus was estimated a minimum sample size of 95 herds of dual-purpose.
Selection of herds. The herds were selected from the data base of project: “Implementación y validación de modelos alternativos de producción ganadera en el departamento del Caquetá” [Implementation and validation of alternative methods for cattle production in Caquetá state], taking into account the 500 herds of dual-purpose cattle distributed in nine municipalities of Caquetá state, and some criteria proposed 22 were used, but modified for this research, thus: a) the farm size (50-180 hectares), b) herds with more than 10 cows in milking (over 36 months of age), c) availability to cooperate in the project, and d) accessibility and roads in good conditions.
Ethics aspects. The samples were taken by Veterinarians and endorsed by the inspectors of the entity of inspection authorized by the Colombian Agricultural Institute (ICA by the acronyms in Spanish) in the Florencia branch, following the ethics, technical, scientific and administrative regulations for the research with animals, according to the law 84 of 1989 of Colombia. Throughout the research the confidentiality of farms positives to BLV was maintained. The project was approved by the resolution 005 of 2013 by the Science, Technology and Innovation Fund (FCTeI) of the National Planning Department (DNP) of Colombia with advice of Colciencias as technical secretariat.
Diagnosis. Between the months of January to March of 2016 blood serum sampling were carried out in 100 herds dedicated to the dual-purpose cattle system, in each were sampled ten bovines (nine cows and one bull) over 36 months of age for a total to 1.000 bovines.
Blood samples were obtained by venipuncture in the coccygeal vein, prior cleaning and disinfection of the area with ethyl alcohol at 70% 23, the blood samples were deposited in sterile tubes without anticoagulant (red top) to ensure that the serum obtained, all samples were properly labeled the animal information: identification, sex, age, identification to herd and date of sampling.
Subsequently, the blood samples were stored in thermos conveyors maintaining refrigeration at 4-8°C. All samples were sent to the laboratory of veterinary Diagnostics of the Colombian Agricultural Institute (ICA) in the Florencia city.
In the laboratory of Veterinary Clinical Diagnostics was conducted the tests established by the ICA for the diagnosis of bovine leukemia virus in Colombia, blood serum was obtained and was carried out with indirect Elisa test for detection of antibodies anti-GP51 of BLV (IDEXX) 11,24. A bovine was considered positive to BLV if the serum-to-positive ratio was lower than 40%, as recommended by the manufacturer of the kit 25.
Data were tabulated in Excel spreadsheet and subsequently analyzed by descriptive statistics using the statistical software InfoStat 26, contingency tables for analysis of categorized data were used, and also, ANOVA was performed with DGC test at 0.05 of significance level as multiple-comparison method 27.
RESULTS
In total were sampled 1000 bovines distributed in 100 herds of dual-purpose cattle, of which 893 blood serum corresponding to cows, 80 to Bulls and 27 to hemolyzed blood serum because they were hemolyzed in the transport.
Sero-prevalence of bovine leukemia virus in the Caquetá state was of the 25.18% (p<0.05, CI: 21.9-28.46), with the equal prevalence both in females as in males (Table 1).
Table 1 Sero-prevalence of bovine leukemia virus (BLV) in the Caquetá State.

Value with same letter does not have statistically significant (p<0.05).
Low sero-prevalence of BLV was found in La Montañita (7.12%), San Vicente del Caguán (14.62%), Albania (21.85%), and Cartagena del Chairá (30.70%) municipalities, and statistical difference in this four municipalities was not found; in the same way, higher sero-prevalence of BLV was found in Milán (30.70%), El Paujil (33.10%), El Doncello (38.92%), San José del Fragua (38.92) and Puerto Rico (41.81%) municipalities, these last five municipalities showed the statistical difference with the first four (Figure 1).
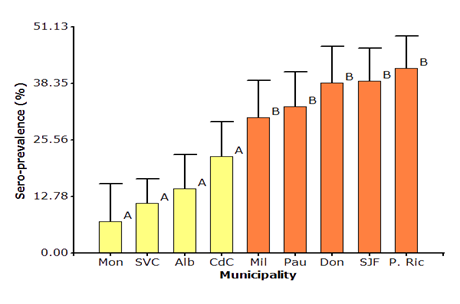
Mon: La Montañita, SVC: San Vicente del Caguán, Alb: Albania, CdC: Cartagena del Chairá, Mil: Milán, Pau: El Paujil, Don: El Doncello, SJF: San José del Fragua and P. Ric: Puerto Rico.
Figure 1 Sero-prevalence of bovine leukemia virus in nine municipalities of the Caquetá state.
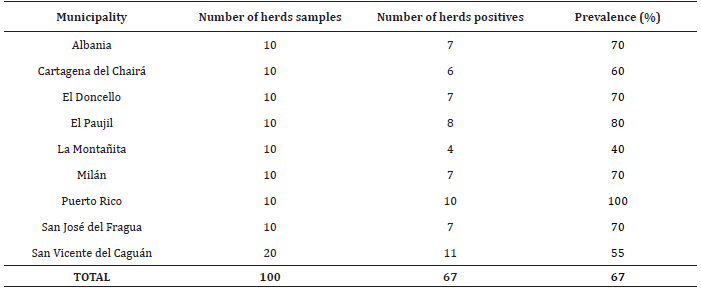
At least one positive case for bovine leukemia virus was criteria for considering a positive herd, were meet in 67 herds of nine municipalities, thus the prevalence of disease at level of herd was of 67% (p<0.05, CI: 57.24-76.76%), (Table 2).
DISCUSSION
In a study by cluster in Colombia 28, found a prevalence for BLV of 22.6%, where dairy cattle were the most susceptible to the infection with 50.7%, followed by the dual-purpose cattle with 14.9%, results lower than this study.
In Colombia, has been reported sero-prevalence of BLV at municipal level, in the following states: Antioquia 44-47.8% 25,29, Boyacá 41.44% 30, Caquetá 11.2% 15, Casanare 15% 31, Cesar 36% 32, Córdoba 21% 33, Cundinamarca 26-64% 34,35, Nariño 19.8% 36, Valle del Cauca 30.3 - 77.83% 24,37, Santander 73.3% 38 and the region of the Magdalena Medio 64.94% 39. In this sense, the report of BLV in Colombia varied between 11.2 to 77.83%, therefore, the prevalence of the disease in Caquetá state (25.18) is between national ranges, nevertheless, it is greater to the prevalence in Casanare, Córdoba, and Nariño, but lower than the others states.
For South America various studies have been performed. In the Barinas state of Venezuela, 23, found a sero-prevalence of 60.83% of BLV, also in Argentina for the year 1999 the sero-prevalence of infected animals with BLV was of 32.85% 40 and for the 2007 seroprevalence was of 77.4% 41, likewise, in Perú, Chile and Bolivia seroprevalence level was of 42.3, 27.9 and 30.7% respectively Polat et al 41 reports were higher than this study. However, in a research in Brazil a sero-prevalence of 11.7% were found 42, being lower than this study.
Worldwide the prevalence of BLV is varied, in studies performed in Asia the prevalence of BLV is higher than this research, for example, in the prefecture of Osaka, Japan, a total of 774 dairy cows over 48 months of age were sampled and the prevalence of BLV was of 60.33% 43, other study in nine provinces of Thailand 44, found a seroprevalence of BLV between 11.0 to 100%. But in a study in four provinces of Cambodia sero-prevalence of the BLV was between 0 to 16.8% 45 and, in four township of Myanmar a prevalence of 9.1% was found 46, being both lower than this research. Nevertheless, in Europe the prevalence is lower than this research, for Italy 47, reported an animal sero-prevalence of 0.015%. In Finland 10, for the year 2001 reported a prevalence of BLV of 0%, and for Portugal until the year 2005 a seroprevalence was of 0.036% 48. For Africa the epidemiological situation is not better, in a study performed in Namibia, a sero-prevalence of BLV was of 12.3%, but varied of 0 to 52.6% at level of districts 49; in six regions of Tanzania a study found a seroprevalence of BLV of 36% 50, contrarily, in two districts of Somalia the sero-prevalence was of 1.5% 51, and other in a province of South Africa was of 12.6% 52, being lower than this study.
Moreover, the presence of BLV depends on the breed, being low in animals of Brahman breed 53, but high in Holstein 40, found that in sero-positive cows to BLV the calving interval is higher than sero-negatives cows. In the same sense, a study performed in Michigan state determinate that an increased prevalence of BLV is associated with decreased herd milk production and decreased cow longevity 56.
At level of herd 54, in the IX region of Chile, found in 279 herds a prevalence of BLV of 59%. In the Mar and Sierra, Argentina, a prevalence was of 68.5% in dairy farms 55. A study developed in Japan found a prevalence of BLV of 44.8% 45. Other study in five prefectures of Japan showed a sero-prevalence at level of herds between 47 to 61% 2. In Argentina the prevalence in herd was of 84% 43. On the contrary, in Italy the prevalence in herds was of 0.08% 49. In Iran, prevalence in farms was of 43.9% 56.
Although it is not clear the capacity of the BLV infect the human through the consumption of products derived from BLV-positive animals, antibodies against BLV have been found in humans, rethinking the hypothesis of a possible zoonosis; indeed, in patients with canicular carcinoma (breast cancer) was found a presence of 7% for protein gp51 of BLV suggesting presence in this pathology 57.
In conclusions the seroprevalence of Bovine Leukemia Virus (BLV) in the dual-purpose livestock over 36 months of age in Caquetá state is moderate with 25.18%, do not exist statistical difference between seroprevalence of cows and bulls. At level of herds the prevalence of BLV is high with 67%. Improving strategies of control and managements in the herds, as well as implement policies of sanitary management are necessary.