INTRODUCTION
The development of effective fertility control methods in animals, especially mammals, is a priority of modern veterinary practice in face of growing need to guarantee animal welfare in every aspect. Livestock systems require the inhibition of male sexual development to shorten the fattening period and ensure proper organoleptic characteristics of meats to cope with market demands 1. Moreover, there is a need to prevent aggressiveness, undesired reproduction, and sexual behavior, which can cause difficulties during husbandry and damages to other animals, facilities, and pasture 2. In turn, the reproductive cycles of females should be controlled according to the goals of production, such as estrus synchronization for artificial insemination 3, anestrus control to regulate pregnancy intervals 4, or prevention of reproduction at the end of the productive cycle. For their part, pet owners and breeders demand of noninvasive, harmless and effective methods to control animal fertility and sexual behavior 5.
Traditionally, the solution to these problems has been male castration based on surgical, chemical, or physical methods and, in females, sexual segregation and ovariectomy. Small pets and animals also underwent surgical sterilization. These methods, however, interfere with animal welfare causing pain, stress, increased risk of infection, diseases, hemorrhagic disorders, and deaths. Their application is impossible in wild and non-stabled animals, and practices like hunting, intoxication, and even poisoning, are performed to control their populations. These inhumane and punishable procedures are unethical, and several animal welfare organizations demand their total elimination.
Consequently, a more tightened legal framework has been established seeking better animal protection. The European Union has banned surgical castration of >7-day-old male animals without anesthesia and analgesics 6,7, but its final objective is to reach the phasing out of surgical castration 8.
In this context, immunization of males with gonadotropin-releasing hormone (GnRH) or immunocastration has occupied a leading place among researches in this field. Active vaccination against GnRH, using hormone analogues or appropriate adjuvants, produces anti-GnRH antibody response capable of neutralizing the circulating hormone. The inability of the hormone to bind to its receptors in the pituitary suppresses the synthesis of gonadotropins, testosterone and inhibin B, causing infertility 9. Immunocastration has shown productive and animal health benefits. However, its wide use in livestock farming is hampered by the laboriousness that implies the application of immunization schemes in large livestock exploitations 10.
Thus, this paper examines the current state of research on fertility control, emphasizing in the area of immunocastration vaccines based on their promising results after application in recent years.
DEVELOPMENT
Today, the alternatives to control animal fertility include surgical castration with anesthesia and/or analgesics, sperm selection to generate gametes of a desired sex, extensive breeding of whole males 11, physical separation of animals of different sexes, hormonal ablation, and vaccines. In practice, surgical castration and vaccines have proven higher feasibility 12.
The vaccines to control fertility pursue two main goals: immunological contraception and immunocastration. The former prevents oocyte fertilization by sperm, or implantation of the fertilized egg through autoimmune response against components of the reproductive system, whereas sexual behavior and mating remain normal. In females, these approaches are useful to control invading, wild or free-living species 13. In the second, the purpose is to eliminate reproduction and sexual behavior in males and females, and it is more commonly used in livestock systems and pets 13. Several veterinary vaccines developed for both purposes, in different species, have been commercialized. (Table 1)
Table 1 Commercialized vaccines for fertility control and immunocastration.
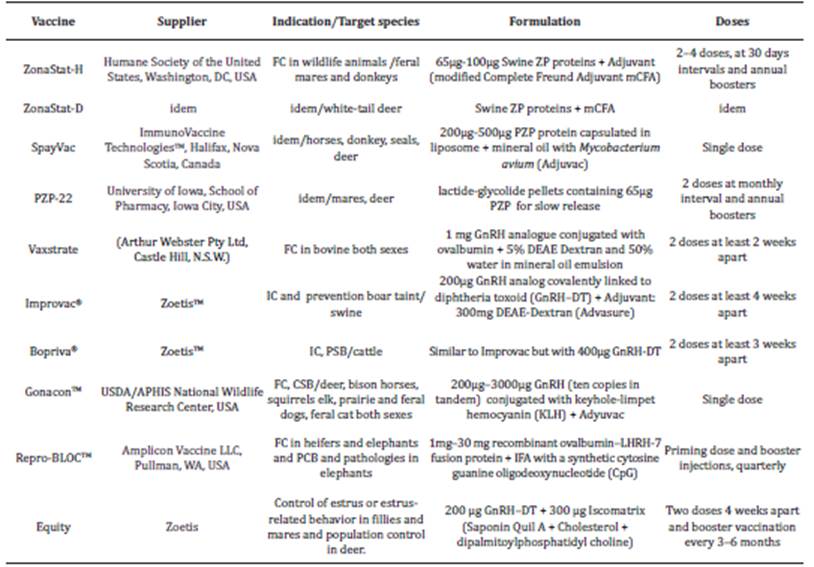
FC-fertility control, IC-Immunocastration, PSB-prevent sexual behavior
Contraception using vaccines against spermatozoa- and egg-specific proteins
The zona pellucida (ZP) contains 3 or 4 proteins, species, identified as ZP1, ZP2, ZP3, and ZP4, with various preservation degrees between the mammals 14, which are attractive targets for immunological contraception in females. These glycoproteins form a matrix surrounding the oocyte, and allow spermatozoa receptivity, the induction of the acrosomal exocytosis at the sperm-binding site, prevent polyspermy, and protect the fertilized oocyte during the initial stages of differentiation 15.
The ZP proteins used as heterologous vaccine antigens are obtained from natural sources or, and more recently, by recombinant DNA techniques or derived synthetic peptides. The anti-ZP antibodies generated by immunizations can block fertilization and/or folliculogenesis and inhibits ovulation as well as the normal development corpus luteum 15.
Four vaccines against ZP are now available at the market (Table 1). The first one, ZonaStat-H, was approved by the US Environmental Protection Agency (EPA), in 2012. The second variant, ZonaStat-D was approved later, in 2017 15. Both contains ZP-swine proteins obtained from natural sources and, require the application of several doses during the first year, and an annual booster to keep infertility in animals 15.
The third variant, Spayvac (Immunovaccine Technology Inc (IVT, Halifax, NS, Canada) contains lyophilized ZP proteins capsuled in liposomes (DepoVax), forming a water-in-oil emulsion, in Adjuvac (National Wildlife Research Center, USA). This platform known as Vaccimax 15 induces a very strong anti-ZP immunological response, a single-dose application has shown high, long lasting effectiveness (13 years) in gray seals, wild horses 16, dama dama, white-tailed deer (Odocoileus virginianus) 17, and other species.
Recently, PZP-22, another variant of this vaccine based on slow-released pellets, was approved (Table 1) 18. Upon administration to mares, the number of parturitions was reduced in 79%. However, its efficacy is being studied, since only a 38% reduction was achieved after the second application, the following year 18.
The main issue associated with these vaccines, is the irreversibility of infertility due to oophorotis and follicular atresia 19. Three additional factors limits its wider use: the species-specific glycosylation of ZP-proteins, the maintenance of estrus and mating rituals among the animals 20 and the extension of breeding season 21 limiting its use in livestock systems and in wildlife.
Despite the identification of about 10 target antigens: sperm-specific lactate dehydrogenase (LDH-C4) 22, sperm adhesion molecule SPAM-1 (PH-20) 23, fertilization antigen FA-1 24, and others; the development of vaccines against sperm proteins has been a more complex endeavor. The goal is to produce antibodies that block the binding of sperm to the oocyte and its fertilization, to cause infertility in males during sperm production in the testicles and its maturation in the epididymis and in females, through interference of sperm-egg interaction in their reproductive tract and the preimplantation embryonic development 25.
LDH-C4 is an enzyme present in the testicles and sperm of birds and mammals, which takes part in energetic metabolism and sperm capacitation. In turn, SPAM-1 24, is a glycoprotein associated to the plasmatic and internal acrosomal membranes of sperm which has hyaluronidase activity 12. Its function is to attach to the ovum and penetrate the cumulus 25. Their combination with cellular structural proteins β-actin and α-tubulin homogenized with N-acetyl-muramyl-L-alanine-disoglutamine hydrate in the presence of CFA or IFA are part of a vaccine candidate used to control fertility in bitches. A phase-1 clinical trial in dogs showed serum antibodies response against surface proteins of spermatozoa LDH-C4 and PH-20, which caused infertility, but also maintained estrus. The authors considered that the presence of anti-actin, anti-PH-20, and anti-tubulin antibodies in the oviductal fluids inhibited capacitation, acrosomal reaction, and attachment of spermatozoa to the ovum, whereas the anti-LDH-C4 antibodies immobilized spermatozoa, inhibiting fertilization 26. The effectiveness of candidate should be evaluated in different species, since formulations based on protein LDH-C4 supplied to female macaques induced high specific antibody titers without causing infertility 27.
Hormones as a targets for immunocontraception.
The need to develop vaccines for fertility control in multiple species and in both sexes, have turn attention of researchers to sexual hormones: follicle stimulating hormone (FSH) and its receptor (FSHR), luteinizing hormone (LH), and gonadotropin releasing hormone (GnRH) 28.
The LH-based vaccine candidates produce specific antibodies that induce azoospermia, suppress testicle function and testosterone production. However, they have been unsuccessful due to their weak immunogenicity and the alopecia and weight loss observed in immunized monkeys 28.
Among hormones and receptors of LH, FSH, the thyroid stimulating hormone (TSH), and chorionic gonadotropin (CG), there is a significant homology in the structures and amino acid sequences 29. Therefore, LH/FSH vaccination and its receptors can induce antibodies toward preserved regions of the homologous receptors interfering in their functions. The vaccination of non-human primates with ovine FSH led to a testicular dysfunction, oligozoospermia, high presence of abnormal spermatozoa in the semen, and infertility, with no decrease in the concentration of serum testosterone 30. However, FSH-deficient male mice have demonstrated to be fertile, despite of decrease in testicle size 30. The most promising results have been achieved with a candidate that requires of an initial immunization with recombinant FSHR, and re-immunizations with a recombinant peptide (32-44aa segment) derived from an immunodominant domain for B-cell recognition of the FSHR which caused infertility in mice without inducing changes or affecting the reproductive apparatus 31.
Moreover, GnRH plays a key role in the regulation of fertility in mammals. It is a well-preserved hormone in vertebrates, which has three isoforms: GnRH-I, GnRH-II, and GnRH-III with different functions. Isoforms I and II induce gonadotropin secretion in mammals. GnRH-I is a decapeptide produced by a limited number of neurons in the hypothalamus, and transported by the hypothalamic-hypophyseal portal system to the anterior pituitary. Then, it binds to the gonadotropin receptors to stimulate LH and FSH secretion into the blood torrent. GnRH secretion through the neurons is pulsatile, thus guaranteeing cyclical production of FSH and LH 32.
The GnRH-based strategies to control fertility have included the utilization of antagonists and agonists of the hormone, GnRH-based peptide vaccines or their receptors, passive immunization, and destruction of receptors using the cytotoxins-coupled hormone. However, considering the scope of this review we will refer only to active GnRH-immunization. Anti-GnRH vaccination includes the production of specific neutralizing antibodies of the endogenous hormone by suppressing the synthesis and secretion of gonadotropins in the pituitary, and consequently, of gonadal steroids, blocking gametogenesis and causing infertility in both sexes 33,34,35.
In the 1980s and 90s, pioneer studies conducted to different mammal species proved that immunizations with native GnRH or analogues (GnRH-based peptides with punctual mutations in the 6th position, particularly) conjugated, chemically, to epitopes or immunogenic proteins and administered in high doses in the proper adjuvant, led to an immunological response against the hormone capable of neutralizing it 33,34. Consequently, testosterone was reduced to castration levels, along with gametogenesis arrest, atrophy of the reproductive apparatus, and prostate hypoplasia 35. These effects were reversible when immunization ceased 35. Nevertheless, the heterogeneity in the intensity of anti-GnRH immune response in animals, and the short duration of immunocastration showed the need for new formulations, dose studies, and more effective adjuvants.
Vaxtrate was the first commercial vaccine for immunocastration (Table 1); it was withdrawn from the market due to its low immunogenicity and adverse effects associated with the adjuvant 36. Today, more advanced GnRH based vaccines are being sold (Table 1). All were designed by conjugating the native or modified hormone with immunogenic proteins and epitopes capable of stimulating T-helper immune response (Th) and generate GnRH neutralizing antibodies using different adjuvants.
The most successful example, of a GnRH-based vaccine has been Improvac (Zoetis, United States) (Table 1) 37. For immunocastration two doses should be administered to pigs that will be slaughtered at 26 or 27 weeks; while three doses should be applied to those that will be slaughtered at older age. After the third dose, castration remains effective for 20 months. The immunocastrated animals have higher contents of lean meat, and larger carcasses than the surgically castrated animals 37. In experimental studies, Improvac has also shown positive results to delay puberty in males 38, whereas caused estrus inhibition in immunized females, along with decreased progesterone levels, and the ovarian and uterine atrophies 39.
Zoetis also has developed Bovipra, a vaccine for bovine castration 40. It is applied to 20- and 25-month-old bulls, resulting in very low testosterone levels for 12-16 weeks. Its application in breeds like Zebu bulls (Bus indicus) or their crossbreds, Holstein, Hereford, Charolais, and Brown Swiss, has produced 80% effectiveness 41.
The most commonly adverse effects observed with both vaccines are pyrexia, transient pain, and swelling at the site of injection 42.
In countries like Brazil, where cattle undergo delayed castration (18-24 months old) to ensure high weight gains, immunocastration has replaced traditional castration to lower costs 1. Its main advantages are associated to prevention of myiasis and infections, improvements on feed conversion, stress reduction, stimulation of growth and weight increase, whereas there is an increment in lean meat contents by animal with a high quality for the industrial processing 1,40,41. Since castration takes place at older ages than in traditional livestock, these animals are superior anabolically, due to keeping high concentrations of anabolic hormones and growth hormones in the serum for a longer time 41.
GonaCon™, is a vaccine (Table 1) conceived to control super populations of several wild mammal species 42. It is available at different peptide doses formulations, depending on the size of the species to be immunized: small animals received up to 400 μg, and large animals, up to 2 mg, intramuscularly. This vaccine has high safety profile and is 100% effective for a year, then this property vary among species, according to the results of clinical trials performed on squirrels, dogs, deer, felids, bisons, and others 42.
No immunocastration and fertility control vaccine for ovine is commercially available 1, though anti-GnRH vaccines used in other species have been administered to male ovine, resulting in a reduction of aggressive behavior, mating odors, testosterone levels, and size of reproductive organs 43. An experiment conducted by Kiyma et al 44 in sheep, demonstrated that immunocastration lowered food conversion efficiency and the weight gain rate, when compared to intact animals.
In recent years, our research team has been working on the development a novel GnRH-based vaccine candidate for immunocastration in livestock systems. The originality of our approach lies in the combined use of two 27-amino acids size GnRH- based synthetic peptides 45. Based in previous studies 46, both peptides, in equivalent amounts (750 μg total), were adjuvated in Montanide ISA 51 VG (Seppic, France) and very small size proteoliposomes (VSSP). Formulation was conceived to induce Th1/Th2 immune responses, taking advantage of adjuvant's ability to produce a powerful antibody response and the VSSP's property to activate the innate immune system 47.
Natural GnRH PEHWSYGLRPG
GnRHml - TT PEHWSYPLRPG GG QYIKANSKFIGITEL
TT - GnRHml QYIKANSKFIGITEL GG PEHWSYPLRPG
Schematic representation of aminoacidic composition of the GnRH analogues peptides including in the vaccine candidate. Both composed by a GnRH-analog obtained by substituting the L-glycine (G) at sixth position in the native hormone by L-prolina (P) (red) fused through a Gly-Gly spacer to a Th epitope (aa 830-844) of the tetanus toxoid (TT) (blue) 47. In one instance, the immunogenic epitope was placed at the carboxyl-terminal end of GnRH (GnRHml-TT) and in the other, the amino-terminal end (TT-GnRHml).
The demonstration of the synergistic effect of both peptides on the production of anti-GnRH antibodies, hormonal ablation and immunocastration (Figure 1) as well as, contribution in the homogenization the anti-GnRH antibodies response and its associated biological effects, among all immunized animals were essential results of the proof of concept carried out with our vaccine candidate 48. On-going preclinical characterization trials might lead to a more innocuous vaccine causing highly effective and long lasting immunocastration.
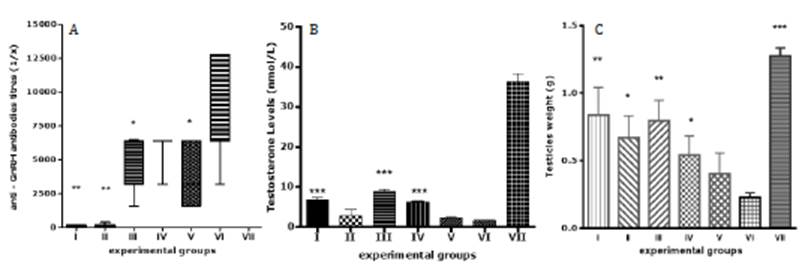
Figure 1 Biological effects 75 days after first immunization with TT-GnRH/GnRH-TT vaccine candidate. Male adult Copenhagen rats (n = 7 per group) received 4 immunizations, fortnightly with following combinations: I- GnRHm1-TT/Montanide ISA 51VG; II- GnRHm1-TT/ Montanide ISA 51VG/VSSP; III- TT-GnRHm1/Montanide ISA 51VG; IV- TT-GnRHm1/Montanide ISA 51VG/ VSSP; V- GnRHm1-TT/TT-GnRHm1/ Montanide ISA 51VG; VI- GnRHm1-TT/TT-GnRHm1/ Montanide ISA 51VG /VSSP; VII-Placebo. In the graph is shown synergistic effect of immunization with both peptides (Groups V and VI) on increasing serum anti-GnRH antibodies titers (A) falling serum testosterone to castration levels (B) and the decreasing of testicles weight (C). The differences among average values of evaluated variables for each group respect to those in Group IV are represents by asterisks. Mann-Whitney test (p<0.05).
Undoubtedly, the use of GnRH-based vaccines in livestock systems benefits to animal welfare. However, stabling and the short time of life of the animals, hamper the assessment of the medium and long-term adverse events associated with these vaccines. In wildlife animals, anti-GnRH vaccination, has been related with changes in its muscular appearance and with the atrophy of some secondary sexual characters such as the elk or deer's antlers and the scent-marking glands in capybaras 49. Hormonal ablation in gregarious animals affects both the structure of the herd and the social interaction between its members. It has been reported that immunocastrated feral horses (E. caballus), feed, rest and travel less 50. The loss of aggressiveness, dominance and hierarchy increasing the vulnerability of vaccinates respect to the rest of the males affecting drastically its survival in the wild.
Long - term immunity and vaccine delivery systems: the two great research challenges in immunocontraception.
There is consensus on the feasibility of oral and oral-nasal routes for immunization of different species in the most diverse habitats. However, such vaccines are complex to develop, since the peptides or proteins required should be resistant to low-pH degradation and the proteolytic enzymes present in the gastrointestinal tract 51. Moreover, problems related to poor oral absorption, low metabolic stability, and difficulty going through the biological membranes of these molecules, should be addressed 52. Additionally, the formulations must be highly stable to allow their administration mixed with meals and, to be released in the extreme environmental conditions proper to natural ecosystems, as well as, provide effective antigenic presentation through the Peyer's patches in the intestines. Several carrier or antigen-releasing systems have been designed in liquid and particulate formulations. These systems, in the form of emulsions, liposomes, microparticles, nanoparticles, or archeosomes (vesicles of polar liquids of halophilic archaebacteria), containing innate and adaptive immune system stimulating molecules, might be a solution to avoid adjuvant use, due to their capacity to enhance the immunological response.
Dendrimers or synthetic self-adjuvating lipopetides are another attractive alternative to develop oral vaccines. Newly developed dendrimers contain various GnRH copies attached to a lipid nucleus derived from lipid A, which is composed of 2-amino-d, l-hexadecanoic acid, and Th and B immunogenic epitopes. The immunization of mice with this molecule produced high anti-GnRH antibody responses, and the dendritic cells stimulation 53. This and other chemical modifications to peptides, like cyclization, glycosilation, and lipidation, are viable alternatives to modulate their physical and chemical properties and increase their bioavailability orally. Oral administration of glycosilated GnRH-analogue peptides to rats has demonstrated their metabolic stability, a capacity to penetrate the cell membranes through glucose transport systems, and high resistance to proteases and the acidic environment of the gastrointestinal system 54. However, the effects of these modifications on peptide immunogenicity should be evaluated.
Chitosan, a low molecular weight cationic polysaccharide, has also been used as adjuvant in a GnRH-based recombinant vaccine to control fertility by inducing long-lasting Th1/Th2 immune response in Balb/c mice of both sexes 55.
Adjuvation with immunostimulating complexes (ISCOMs) (lipophilic saponins that form a box-like structure with the antigen) is another alternative for GnRH-KLH immunization. Preclinical studies shown the induction of elevated anti-GnRH response in immunized mice 56.
Recently, high molecular weight polyethylene implants containing GnRH attached to multiple antigenic peptides (MAP) or to covalent dendrimer peptides loaded with nanoparticles of polyanhydric co-polymers resembling bars have been developed. Upon implantation in C3HeB/FeJ mice, B and T immune responses were induced for 41 weeks but failed to produce infertility 57.
The utilization of self-replicating releasing systems based on non-pathogenic bacterial or viral vectors, such as lactobacillus, vaccinia viruses, myxoma or murine cytomegalovirus, etc., to design oral or oral-nasal vaccines to control fertility for a long period is appealing, due to their inter-species specificity and their capacity to replicate in the host. A review of Cross et al 58 tackles preclinical results of immunization of different species with candidates based on recombinant viral and bacterial vectors expressing different ZP antigens administered through various routes, including orally.
The use of the so-called recombinant adeno-associated viruses (AAV) has been evaluated to design of vaccines to control fertility. This is a group of single-chain DNA viruses, from vertebrates, belonging to family Parvoviridae whose genome (surrounded by a non-envelope capsid) only has two genes: rep (replication, integration), and cap (structural), as well as transgenic and regulating sequences flanked by two inverted terminal repeats. The integration frequency of these non-autonomous systems is very high, but they cannot replicate unless there is an infection with adenovirus or other viruses. Their advantage is that they can be expressed in non-dividing cells for a long time. The main disadvantage of these systems is the heterogeneity of the auto-immune response caused by low-level transgene expression, which hinders effective presentation of the antigen to the immune system. For instance, in a recent study mice of both sexes were inoculated intramuscularly with 109-1011 AAV particles expressing a recombinant anti-GnRH antibody; only the ones that received the highest dose witnessed infertility for 52 weeks 59. Additionally, the administration route, the risk of affecting non-target species, and their irreversible effects are significant downsides.
In our point of view, the development of edible formulations that minimize the animal handling could be the turning point for the massive use of immunocontraception in veterinary practice. In turn, antigenic composition of vaccines will vary according to needs; the immunocastration, contraception in females or fertility induction. For instance, GnRH-based vaccines will be of choice for fertility control in livestock systems and stray animal populations, as they suppress the sexual and aggressive behavior of species, facilitating their management. However, to controlling fertility of wildlife species, where their survival depends on physical performance, the maintenance of rituals associated with reproduction and dominance as well as the social structure of herds, vaccination of the females against sperm proteins could be the best option. The control of fertility in pets is another very complex research field, where the search of solutions satisfying the owners, breeders and guarantee animal welfare, is required, thus demanding vaccines with a high safety profile and long-lasting, reversible effects.
CONCLUSIONS
The application of immunological methods to control fertility is spreading in common veterinary practice, contributing a reduction in animal suffering. However, their use is not exempt from ethical issues because of limit the natural behavior of species. Hence, it is important to design on new products adjusted to each species' habitat and needs, which poses new challenges to researchers.
Considering on-going research, the problems observed in these vaccines in terms of specificity, heterogeneity and duration of the immunological response, the utilization of multiple doses and administration routes depending on habitat diversity, the requirements of animal welfare and handling, and high costs, might be solved in the near future. Nevertheless, in the application of the new products in veterinary medicine practice there should prevail a preservationist approach toward the protection of ecosystems and ensure the existing balance and welfare among species as the only way to prolong life on the planet.















