INTRODUCTION
Colombia ranks first regarding the number of bird species in the world (+1900), and the Andean regions have stood out for their high levels of biodiversity, threat, and endemism 1,2,3,4,5. More than 1500 bird species are found in the Colombian Andes, representing 84% of the country's total and more than 15% of the world's species 4,5. The Andean physiography has promoted that the local bird diversity (alpha) of a certain slope is low since the richness of species decreases with altitude 4; however, due to the species' exchange among latitudinal and watersheds assemblages, the beta diversity is found on a large scale, allowing the maintenance of high regional diversity (Gamma; 6,7,8,4). The Andes is a region where more than 70% of the country's human population is concentrated; therefore, it exhibits a high level of deforestation and changes in land use 9,10,11. Thus, this highlights the importance of promoting management plans and conservation strategies for multiple ecosystems 12.
Wildlife reserve areas are an important source for local biodiversity conservation 13,14, where management plans may promote the maintenance of ecosystems under legal figures that limit the change of land use, restricting and/or regulating the exploitation of natural resources such as hydric soils, plants, and animal species 14,15. The important bird areas (IBAs) are an initiative that is promoted worldwide for the identification and declaration of globally or nationally threatened bird habitats, sites of endemism, or congregation of species as areas of special importance to implement actions for conservation and research on wealth and status of bird populations 16,17. These areas advocate a protection figure not only for birds but also for most animals and plants coexisting at these sites 13,14.
In Colombia, 124 IBA sites exist currently, and many of these areas were and are used for resource exploitation such as cattle raising, monocultures, and tourism 16. In the Quindío Department, La Patasola is the IBA site with more than 169 bird species reported since its recognition 17. It is located in a montane forest over 2000 m.a.s.l., and it is surrounded by grasslands and monocultures, which are a product of the change in the use of land of previous managements 17,18. However, studies concerning birds in La Patasola have not emphasized the quantification of the structure or the composition of the assemblages present there, and they have not dealt with the possible spatial distribution of trophic guilds between areas of mature forest and its edge.
The objective of this study was to quantify the potential structure and composition of assemblages of birds in two types of habitats (the interior and the edge of the mature montane forest), according to one high rain season in the natural reserve and IBA site La Patasola, located in the buffer zone of Los Nevados National Natural Park 17, using methodologies based on true diversity 19 and sampling coverage 20,21. Additionally, this study aimed to determine if trophic guilds distribution depends on the habitat type.
MATERIALS AND METHODS
Study area. The IBA reserve La Patasola is located in the Northeast of the department of Quindío, on the western slope of the Central Colombian Andes, in the municipality of Salento (04°41'22.49" N, -75°33'0.528" W; 5; Figure 1), and it borders toward the north and the west with the Otún Quimbaya Flora and Fauna Sanctuary (Risaralda Department). The La Patasola has a territorial extension of 130.86 Ha, and it is in the lower montane life zone between 2150 and 2600 m.a.s.l. The average temperature is 17°C, and the relative humidity measures 75% with 2600 mm of annual precipitation 16; moreover, the high rain seasons occur in the months of March to May and September to November 22. The reserve is in an area of very humid, low montane forest, where the landscape is covered by areas of intervened mature forest, advanced secondary forest predominates, and early succession; additionally, all this is surrounded by commercial plantations of Pinussp. and Eucalyptussp.23,18,16.
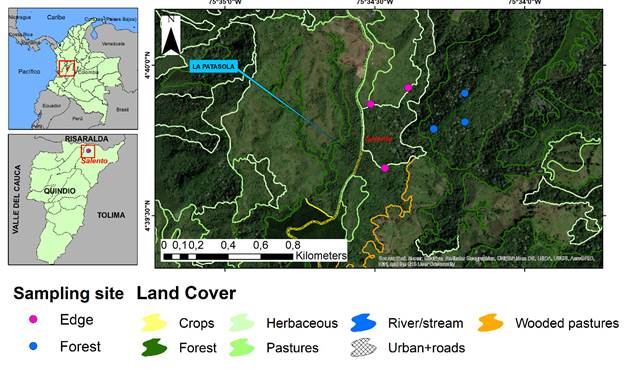
Figure 1 Location of the study area, IBA reserve La Patasola, in the department of Quindío, Central Andes of Colombia, where the fixed sites for censuses in the inner part (blue points) and the edge (pink points) of the montane forest are shown.
This study was conducted in the following two habitat types: (1) the inner mature forest (Forest) with a canopy of 1520 m, in which we recorded most of the individuals of the following species: Nectandra sp., Cecropia telealba, Wettinia kalbreyeri, Guettarda sp., Brosimum utile, Ocotea sp., and Otoba lehmanii; moreover, it is defined as the forest with a distance of 400 m from the edge, and (2) the edge of the forest (edge) as a mixing part of the coverages of the mature forest (Gallery forest) and the wooded pastures and bushes; moreover, it is a trail with a distance of 25 m maximum from the start of the wooded pastures to the forest (Figure 2).

Figure 2 Land cover of two kinds of habitats for birds, in which the left image = edge of the forest and the right image = inner part of the forest, both in the IBA site La Patasola, Central Andes of Colombia.
Methodology. During April of 2016 (high rain season), 12 days of field trip were made over the two habitat types, and sampling was carried out during the first hours in the morning, between 5:00 and 8:00, during which five people conducted censuses from fixed points. These were carried out taking into account points with a radius of 25 meters each (1 point every 200 meters) for a total of 10 in the inner part and edge of the forest (adapted and modified from: 24,25,26). For each species, individuals were recorded separately inside and outside the fixed radius (25 m), and at each point, the census was carried out for 2 hours, measured with a stopwatch, making records either visually or aurally of the species 25.
Observations were made with 8x * 40mm binoculars, and the species were identified with the help of the Colombian Bird Guide of 1986 27 and a Photographic Guide of La Patasola Nature Reserve by Arbeláez-Cortés 18. All birds were associated with one trophic guild 28,29, and the taxonomy list was based on the South American Classification Committee 30.
Data analysis. Birds were categorized according to trophic guilds in the two habitat types (inner part and edge of the forest) as the guilds may represent the functional relationships of group species in order to exploit the same class of environmental resources 31,32,33,29. The categories were made according to the classification of González-Salazar et al., 29, which considers where birds mainly obtain food (i.e., ground, arboreal, air under canopy). The categories are presented as follows: Carnivores (CA), Frugivores (FRU), Granivores (GRA), Insectivores (INS), Frugivores-Insectivores (FRU-INS), Nectarivores (NE), Scavengers (SCA). To determine whether the distribution of birds by trophic guilds was dependent on any habitat, a Chi-square analysis was performed 34.
The diversity of species was compared in both habitats with a sampling coverage analysis 21, and the comparison was performed on the free software R v. 4.0.0 35 within the iNEXT package 36. The sampling coverage varies between 0 and 1, indicating the likelihood that the next randomly captured individual belongs to a species already recorded in the sampling. Levels of sensitivity to the relative abundance of the species are expressed in the following three orders: q = 0, q = 1, and q = 2. When q = 0, the diversity calculations ignore the abundance for each species (Pi), and the diversity value obtained is equivalent to the richness of the species. When q = 1, the species are weighted according to their relative abundance and the analysis corresponding to the exponential of the Shannon-Wienner index 37,38. When q = 2, the diversity results are mainly influenced by the most abundant species and the calculation corresponding to the inverse of the Simpson index 37.
A rank-abundance curve was used to determine the patterns of the distribution of the abundance of the bird species in both the inner part and the edge communities, following the methodology proposed by Magurran 39, in which the ordinate axis (the one containing abundances) is expressed in terms of the proportion with which the species contributes to total abundance (Pi = number of individuals of species i/number of individuals of all species).
The similarity in the species composition of communities was determined by the Chao-Jaccard index 40,41, which was calculated in Estimates v. 9.0 42. Chao-Jaccard index allows to correct biases in sample size and by the absence of records of rare species. Furthermore, it calculates beta diversity using the index proposed by Jost 19, which modifies between 1 when the assemblies to be compared are identical and N (number of assemblies) when they are totally different.
Finally, the beta diversity was divided into two separate components 43, and this method divides the pairwise Sørensen disparity between two communities (βsor, equation 1) into two components that represent the following: species spatial turnover (βsim, equation 2) and nestedness-resultant dissimilarities (βsne). Simpson's dissimilarity index (βsim) describes species turnover without considering the influence of richness gradients 44,45,46,47. Since βsor and βsim are equal in the absence of nestedness, their difference is a net measure of the nestedness-resultant component of beta diversity; thus, βsne = βsor - βsim. The equations for pairwise disparity indices are the following:
Equation 1. βsor = (b+c)/(2a+b+c)
Equation 2. βsim = (min(b,c))/(a+min(b,c))
In the above equations, a = number of species presented in both communities, b = number of species presented only in the first community, and c = number of species presented only in the second community 47. With the resultant values of indexes, the proportion of the nestedness-resultant component to overall multiple site dissimilarity was obtained to represent the relative contribution of overall beta diversity: βratio = βsne/βsor. Thus, βsor < 0.5 indicates that beta diversity is determined dominantly by species turnover, and βratio > 0.5 indicates that nestedness is the dominant component 47.
RESULTS
In total, 80 bird species, which belong to 28 Families and 13 Orders, were recorded, and the Family most represented was the Thraupidae with 13 species while the most diverse Order was the Passeriformes with 51 species. According to IUCN 48, four species belonged to any threatened category as follows: the Odontophorus hyperythrus, Andigena nigrirostris, and Saltator cintus belonging to the Near Endangered (NE) category and the Penelope perspicax, which is also an endemic species of the Colombian Andes, belonging to the Endangered (EN) category (Table 1, Figure 3).
Table 1 The number of bird species by the community of the inner part and edge of the forest (inner, edge), found in the IBA reserve La Patasola.
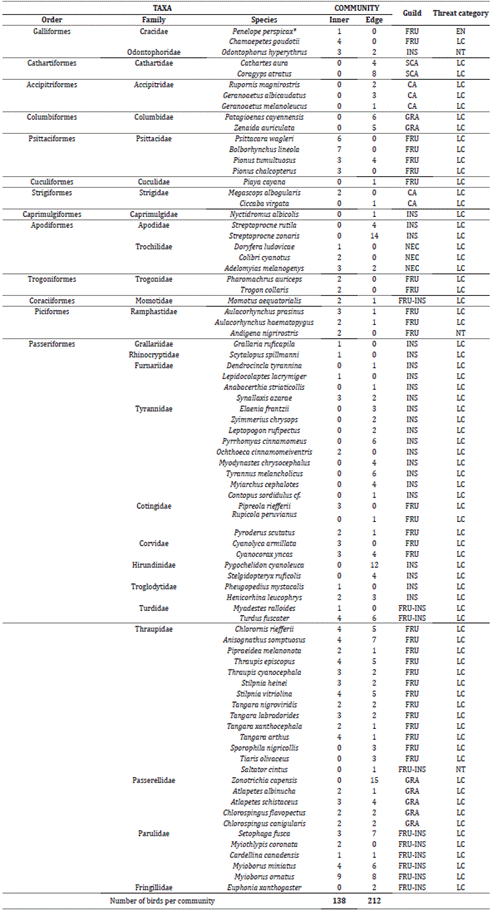
Trophic guilds: CA = carnivore, SCA = scavenger, FRU = frugivore, FRU-INS = frugivore-insectivore, GRA = granivore, INS = insectivore, NEC = nectarivore. Threat category: EN = Endangered, LC = Least concern, NT = Near-threatened. Asterisk shows endemic species to the Andes.

Figura 3 Photographic record of some birds in Patasola: A. Rupornis magnirostris, B. Megascops albugolaris, C. Pyroderus scutatus, D. Turdusfuscater (male), E. Chamaepetesgoudotii, F. Pyrrhomyas cinnamomeus, G. Tangara arthus, H. Geranoaetus albicaudatus, I. Turdusfuscater (female)".
The trophic guild with the highest number of species was the FRU with 20 species in the edge of the forest and 26 species in the inner part of the forest, while the CA guild presented the lowest number of species per habitat, recording only two species in the edge and zero in the inner part of the forest. Furthermore, no significant differences were found in the distribution of trophic guilds in the habitats (Chi-square = 9.1457, df = 6, p-value = 0.1515, Figure 4). Thus, this demonstrates that the distribution of birds according to guild is independent of the area of the forest (whether the inner part of or the edge of the forest).
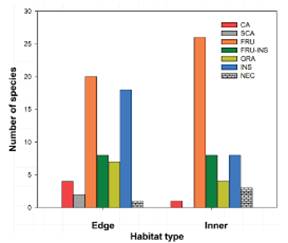
Figure 4 Distribution of bird's trophic guilds on the forest habitats; where CA = carnivore, SCA =scavenger, FRU = frugivore, FRU-INS = frugivore-insectivore, GRA = granivore, INS = insectivore, NEC = nectarivore.
Diversity measures showed that sampling coverage was over 0.90 for both communities (Figure 5). Order diversity of q = 0 was not statistically different between the inner and edge communities; furthermore, order diversities of q = 1 and q = 2 presented to be too alike and not statistically different between communities (Figure 6). Although the greatest abundance was recorded for the Myioborus ornatus species (n = 9) in the inner community and the Zonotrochia capensis (n = 15, Table 1) in the edge community, both assemblage structures of the communities presented similar heterogeneity according to the relative abundance of species (Figure 7).
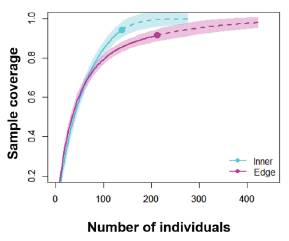
Figure 5 Relationship of sample coverage and number of individuals of bird species between the inner and edge communities.
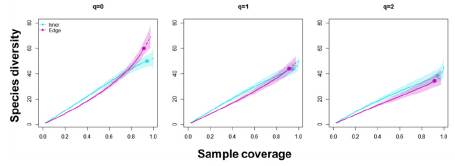
Figure 6 Relationship between the sampling coverage and the diversity of bird species in the inner and edge communities. The "q" values indicate the sensitivity level of the calculations of diversity to the relative abundance of the species (See Methodology). Shaded areas indicate the 95% confidence intervals for each community.
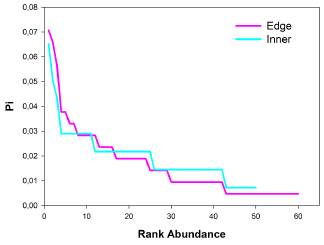
Figure 7 Rank-abundance graph that shows differences in the assemblage structure of birds in the inner and edge communities. Pi = number of individuals of species i/number of individuals of all species.
When calculating the exchange of species between habitats, an index value of beta diversity = 0.626 was obtained, showing that entities (inner and edge communities) are presented as two well-differentiated groups. Disparity index results for βsor = 0.454; βsim = 0.4 and βsne = 0.054; thus, with a βratio = 0.12, this analysis suggests that differences in species composition between habitats were due to a dominant species turnover.
DISCUSSION
Although sample coverage was over 0.90 for both habitats, the analysis suggests that diversity may increase. Based on the relationship between richness of species-area size 49, it is known that sites with difficult access such as cliffs in the forest may exhibit an increased diversity compared to this study 12. Additionally, out of 169 reported birds in La Patasola with an area of 130 Ha, two habitats of montane forest with an area of at least 15 Ha were sampled, which constitutes a little more than 10% of the total territory of the site, and even this gives us a representation of more than the 47% of the expected birds (169 species, 18). The presence of species such as the Penelope perspicax, Odontophorus hyperythrus, Andigena nigrirostris, and Saltator cintus found in this work, in addition to other species such as Leptosittaca branickii and Chlorochrysa nitidissima, reported by Renjifo, et al 50 and Arbeláez-Cortés 18, which occur in any threat category of the IUCN 48, demonstrates the importance for conservation that implies La Patasola as a refuge of bird diversity in the Central Andes of Colombia.
No dependency was found among the distribution of birds by guilds over the inner part and the edge of the mature forest, indicating the potential resource availability for every group (guild) of birds in both habitats even when some groups such as FRU and FRU-INS tend to have a greater number of species and individuals per species by means of the resource representation in forest ecosystems 51,52,53. On the other hand, the SCA guild was only found at the edge of the forest, which can be a normal result since scavengers are frequently observed in open areas, and due to acute eyesight, they exhibit the ability to perform long-distance movements and the capacity of transferring information on carcass location 54,55,56,57.
We expected a greater diversity of birds in the inner part of the forest due to the vegetal complexity 58,59,28. However, at the edge of the forest, we observed 10 more species and 74 more individuals, which presented a slightly major diversity in richness according to q = 0. On the other hand, both habitats seem to maintain the same levels of equity (q = 1) and dominance (q = 2), which can be explained by the fact that some shared species of the families Thraupidae, Paruliidae, and Tyraniidae demonstrate a similar number of individuals; additionally, in both habitats, we did not record a single species with a significantly greater number of individuals. The above is related to the assemblage structure of birds according to the relative abundance of species, which were presented as heterogeneous in both habitats since not one single species was predominant in any habitat. It is possible that even if the same birds were not found in both the inner part and the edge of the forest, other taxons with a similar relative abundance occur in both habitats 60. For instance, this study ascertained that the most abundant species are the Zonotrichia capensis, Streptoprocne zonaris, and Pygochelidon cyanoleuca with 41 individuals in the edge of the forest, representing 19% of the total species of their habitat, and this is similar to the three species Myioborus ornatus, Bolborhynchus lineola, and Psittacara wagleri that recorded 22 individuals in the inner part of the forest, representing 16% of the total species of their habitat.
The exchange of species (beta diversity) showed that the inner part and the edge of the mature forest are presented as two differenced communities since similarity distance measured over 0.6; furthermore, the βratio = 0.12 indicates that the ratio process of a species spatial turnover contributed more to the beta diversity of general communities than a nestedness-resulting process 47, and the lower value of βratio is explained by the strong ability of birds to disperse, not being affected by barriers (as other vertebrate groups), and to find the appropriate habitats in a region (i.e. the inner part or edge of the mature forest) 9,61,62,47. Furthermore, this is evidence of a smaller risk concerning the local extinction of species and the main potential for conservation value of birds in the Andes 9,47.
Although the land cover of the IBA site La Patasola has suffered changes in its composition and structure due to anthropogenic processes, which has led some forest patches to ecological successions, this study has shown how some attributes of the bird's assemblages between communities such as the spatial turnover of species may maintain their local diversity in the montane forest and its edge at least. Furthermore, these results support how La Patasola functions as a refuge area for birds and a place to consider in management and conservation plans of threatened and endemic species of the Colombian Andes, and a prime example of these species is Penelope perspicax.















